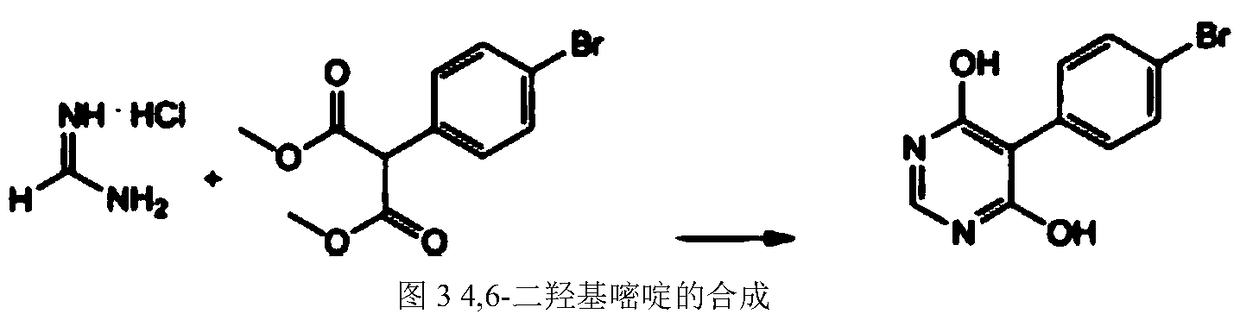
Synthetic method for macitentan drug intermediate
A synthesis method and technology of macitentan are applied in the field of synthesis of macitentan pharmaceutical intermediates, which can solve the problems of high price and low purity, and achieve the effects of increasing concentration and reducing synthesis cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
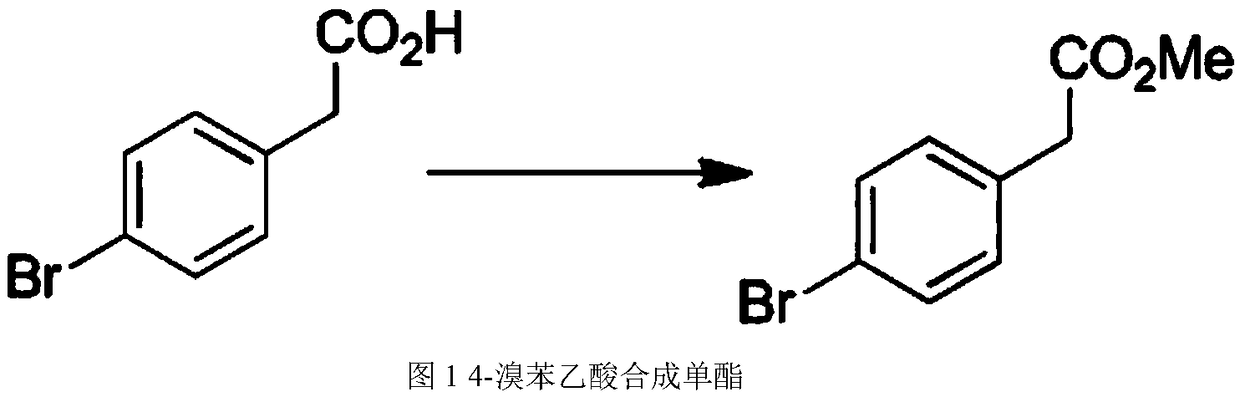
[0019] (1) Synthesis of monoester: 20 parts of anhydrous methanol, 6 parts of 4-bromophenylacetic acid and 8 parts of sulfonyl chloride are completely mixed in a reactor. After the preliminary mixing, place in the lifting digital control thermostat, set the temperature at 70°C, and slowly turn on the magnetic stirrer. After 4 hours of reaction, the reaction process was terminated. The final product obtained by the reaction was dissolved in ethyl acetate, washed with saturated sodium bicarbonate solution, water, and saturated saline, and finally added with anhydrous sodium sulfate to seal and dry. After drying, the solvent was distilled off under reduced pressure to obtain a colorless transparent oil (monoester).
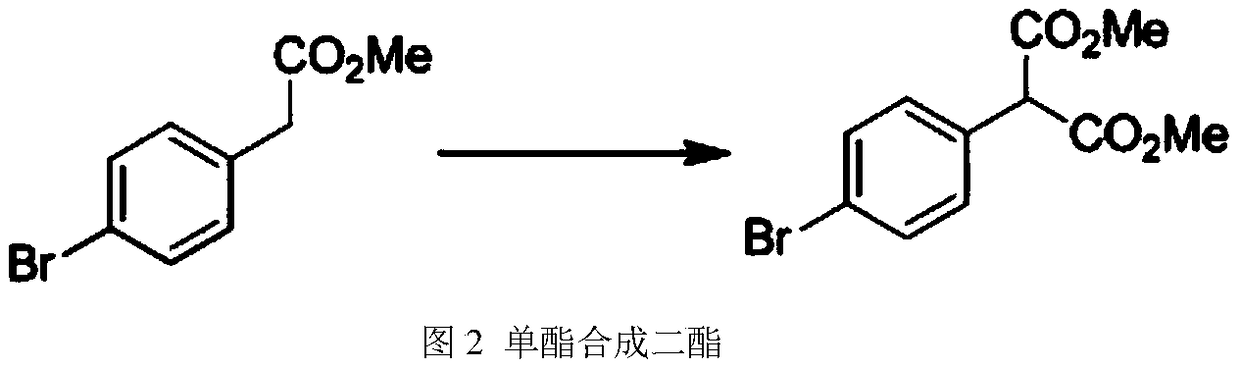
[0020] (2) Synthesis of diesters from monoesters: After initially mixing 10 parts of monoesters and dimethyl carbonate, the reaction bottle was placed in a lifting numerical control thermostat, and the magnetic stirrer was slowly turned on, and the newly prepared so...
Embodiment 2
[0024] (1) Synthesis of monoester: 25 parts of anhydrous methanol, 7 parts of 4-bromophenylacetic acid and 9 parts of sulfonyl chloride are completely mixed in a reactor. After the preliminary mixing, place in the lifting digital control thermostat, set the temperature at 70°C, and slowly turn on the magnetic stirrer. After 4 hours of reaction, the reaction process was terminated. The final product obtained from the reaction was dissolved in ethyl acetate, washed with saturated sodium bicarbonate solution, water, and saturated saline, and finally added with anhydrous sodium sulfate to seal and dry. After drying, the solvent was distilled off under reduced pressure to obtain a colorless transparent oil (monoester).
[0025] (2) Synthesis of diesters from monoesters: After initially mixing 12 parts of monoesters and dimethyl carbonate, place the reaction bottle in a numerically controlled thermostat. Slowly start the magnetic stirrer, add 30 parts of newly prepared sodium meth...
Embodiment 3
[0029] (1) Synthesis of monoester: 30 parts of anhydrous methanol, 8 parts of 4-bromophenylacetic acid, and 10 parts of sulfonyl chloride are completely mixed in a reactor. After preliminary mixing, place in a lifting digital control thermostat, set the temperature at 70°C, and slowly turn on the magnetic stirrer. After 4 hours of reaction, the reaction process was terminated. The final product obtained by the reaction was dissolved in ethyl acetate, washed with saturated sodium bicarbonate solution, water, and saturated saline, and finally added with anhydrous sodium sulfate to seal and dry. After drying, the solvent was distilled off under reduced pressure to obtain a colorless transparent oil (monoester).
[0030] (2) Synthesis of diesters from monoesters: After initially mixing 15 parts of monoesters and dimethyl carbonate, the reaction bottle was placed in a lifting numerical control thermostat, and the magnetic stirrer was slowly turned on, and newly prepared sodium met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com