A kind of synthetic method of riociguat intermediate
A synthesis method and intermediate technology, applied in the field of synthesis of riociguat intermediates, can solve problems such as high risk factor, difficult purification, and many impurities, and achieve the goals of reducing equipment requirements, increasing reaction yield, and simplifying process operations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
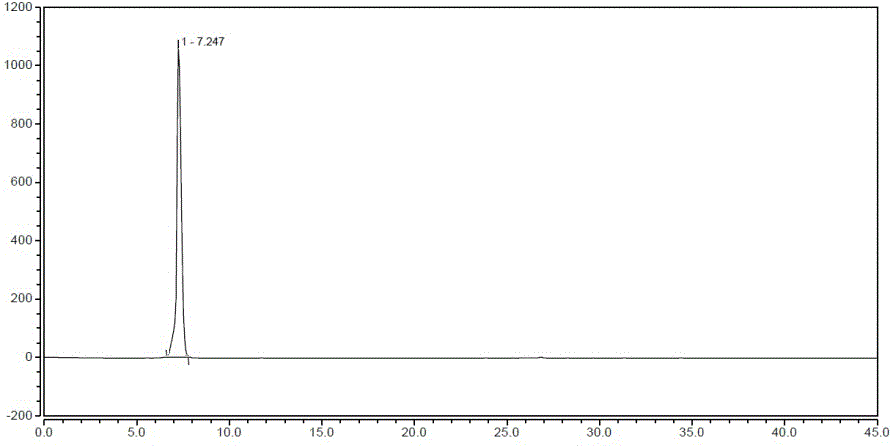
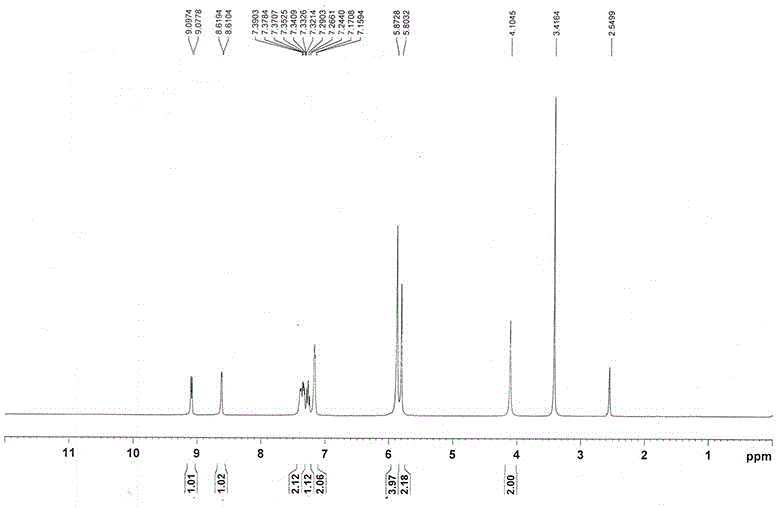
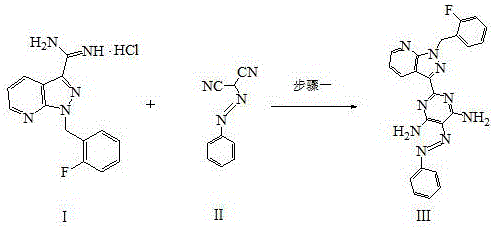
[0036] a. Add 50.0g (0.16mol) 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, 27.2 g (0.16mol) to a 500ml reaction flask mol) phenylpropazomalononitrile, 8.6g (0.16mol) sodium methoxide and 350ml DMF, stirred at 110°C for 12 hours, cooled to 25°C, filtered to obtain a solid, stirred and washed with 250ml of ethanol for 10min, and filtered to obtain a solid , and repeated the above washing steps twice to obtain a solid, which was dried in a drying oven at 85 °C for 5 h to obtain 60.7 g of compound III with a yield of 84.6%;
[0037] b. Add 60.7g (0.138mol) of compound III, 7.4g (0.138mol) of ammonium chloride and 730ml of ethanol / water into a 1 L reaction flask, add 5.8g (0.1mol) of iron powder under stirring, and heat at 79°C After stirring and reacting for 8 hours, cool down to 25°C, filter to obtain the filtrate, distill under reduced pressure (vacuum degree: 0.090 MPa, temperature: 65°C) to obtain the residue, add 120ml of water to the residue a...
Embodiment 2
[0039] a. Add 50.0g (0.16mol) 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, 29.9 g (0.176 mol) phenylpropazomalononitrile, 21.8g (0.32mol) sodium ethoxide and 500ml DMF, stirred at 120°C for 15 h, cooled to 20°C, filtered to obtain a solid, stirred and washed with 250ml of ethanol for 10 min, filtered A solid was obtained, and the above washing steps were repeated twice to obtain a solid, which was placed in a drying oven at 80°C for 5 h to obtain 57.4 g of compound III, with a yield of 80.0%;
[0040]b. Add 57.4g (0.13mol) of compound III, 8.3g (0.156mol) of ammonium chloride and 860ml of ethanol / water into a 1L reaction flask, add 6.6g (0.117mol) of iron powder under stirring, and put After stirring and reacting for 10 h, cool down to 30 °C, filter to obtain the filtrate, distill under reduced pressure (vacuum degree: 0.090 MPa, temperature: 70 °C) to obtain the residue, add 120 ml of water to the residue and stir for 30 min, and filter to obta...
Embodiment 3
[0042] a. Add 50.0g (0.16mol) 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, 33.4 g (0.196 mol) phenylpropazomalononitrile, 5.9g (0.245mol) sodium hydride and 250ml DMF, stirred at 115 °C for 10 h, cooled to 30 °C, filtered to obtain a solid, stirred and washed with 250 ml of ethanol for 10 min, and filtered to obtain a solid , and then repeated the above washing steps twice to obtain a solid, which was placed in a 90 °C drying oven for 5 h to obtain 59.8 g of compound III with a yield of 83.4%;
[0043] b. Add 59.8g (0.136mol) of compound III, 10.9g (0.2mol) of ammonium chloride and 598ml of medicinal ethanol / water into a 1L reaction flask, add 7.6g (0.136mol) of iron powder under stirring, and After stirring and reacting at ℃ for 6 hours, cool down to 20 ℃, filter to obtain the filtrate, distill under reduced pressure (vacuum degree: 0.090 MPa, temperature: 60 ℃) to obtain the residue from the filtrate, add 120ml of water to the residue and stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com