Riociguat preparation method
A technology of 4-b and pyrazolo, which is applied in the field of drug synthesis, can solve problems such as low process yield, difficulty in removing impurities, and potential safety hazards in process production, and achieve simple process operation, improved yield and purity, and easy The effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: LA-1 preparation
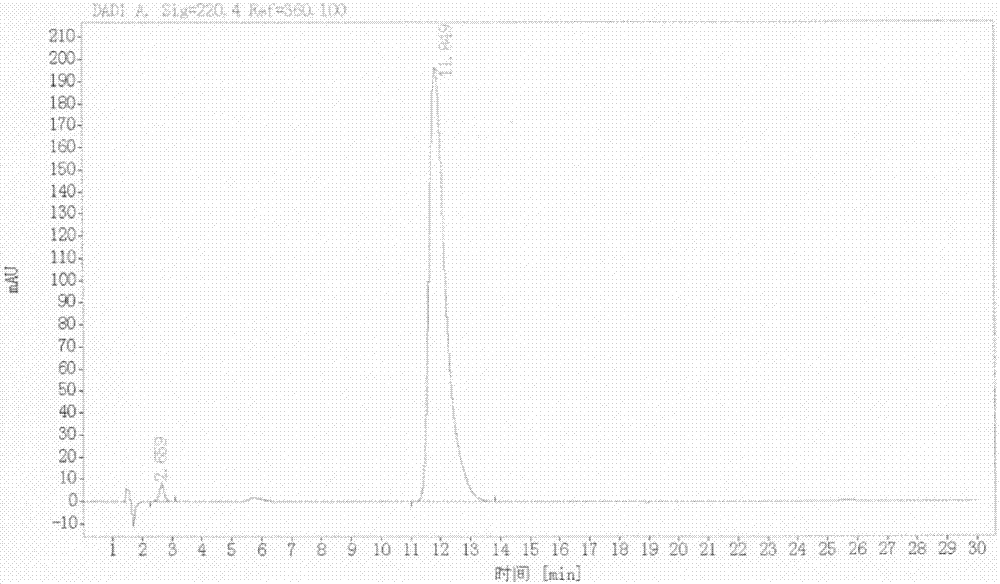
[0063] Add 200ml of dioxane to a 500ml four-necked flask, and add LA-A (200.0g, 0.65mol, 1.0eq), LA-B (110.0g, 0.65mol, 1.0eq), sodium methoxide (70.0g , 1.3mol, 2.0eq), heated up to about 100°C, slowly refluxed, and reacted for 1.0h under stirring. After the reaction, the reaction solution was gradually cooled to room temperature with stirring, and continued to stir for 2.0 h in an ice bath. The reaction solution was suction filtered. Filter with suction, and dry the filter cake at 50°C for 12-16 hours. Obtain brick red solid LA-1: 27.97g (yield 97.4%, liquid phase purity 99.53%, melting point>250°C)
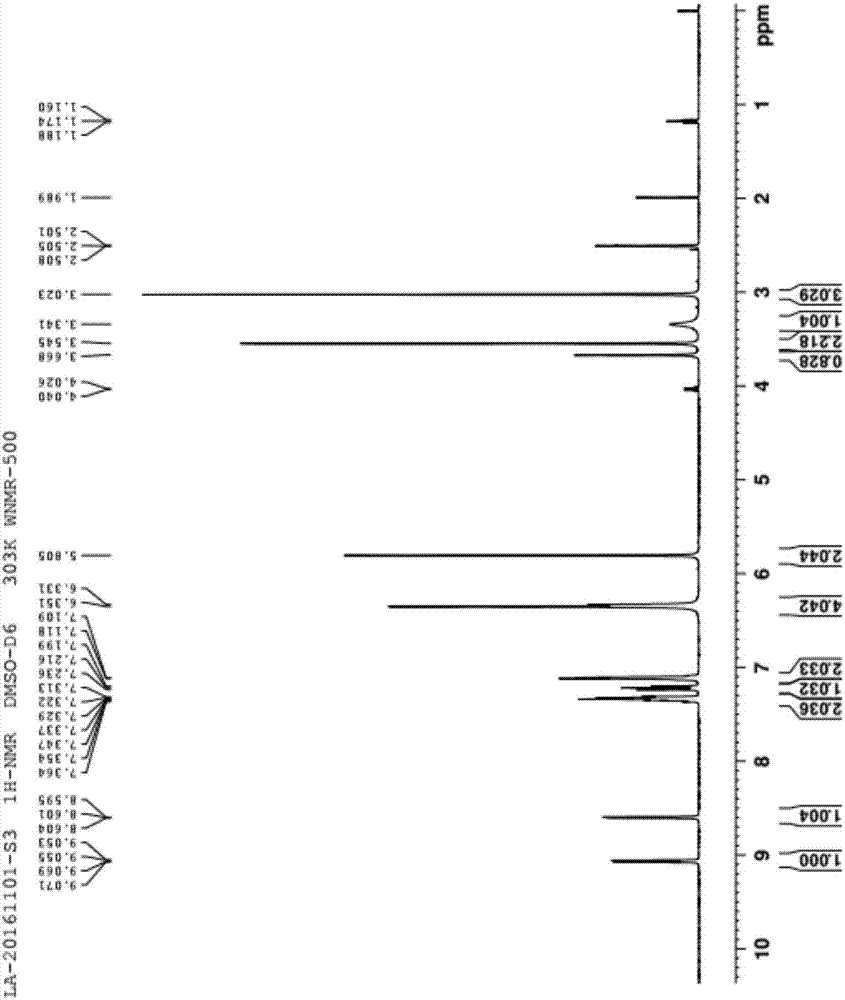
[0064] 1 H-NMR (DMSO-d 6 ): δ=5.850(s, 2H), 7.136~7.166(m, 1H), 7.183~7.257(m, 2H), 7.345~7.418(m, 3H), 7.478~7.509(t, 2H), 7.856(s , 2H) 8.003~8.019(d, 2H), 8.418(s, 2H) 8.468~8.660(dd, 1H), 9.192~9.211(dd, 1H)
Embodiment 2
[0074] Embodiment 2: LA-1 preparation
[0075] Add 60ml of methyl isopropyl ketone to a 100ml four-necked flask, and add LA-A (6.0g, 0.020mol, 1.0eq), LA-B (3.3g, 0.020mol, 1.0eq) and sodium methoxide sequentially under stirring (2.1g, 0.039mol, 2.0eq), heated up to 100°C, and reacted for 2.0h under stirring. After the reaction, the reaction solution was gradually cooled to room temperature with stirring, and continued to stir for 2.0 h in an ice bath. The reaction solution was filtered with suction, and the filter cake was suspended and washed with 60 ml of water. Suction filtration, the filter cake was dried at 50°C for 16h. Obtain brick red LA-1: 7.3g (yield 85.1%, liquid phase purity 99.13%, melting point>250°C)
Embodiment 3
[0076] Embodiment 3: LA-2 preparation
[0077] Add DMF (30ml) into a 100ml four-necked flask, and add LA-1 (5.0g, 0.012mol, 1.0eq), activated carbon (0.5g), FeCl 3 ·6H 2 O (0.8 g, 0.003 mol, 0.25 eq). The temperature was raised to 60° C. under stirring, and 80% hydrazine hydrate (15.0 g, 0.238 mol, 10.0 eq) was slowly added dropwise. After the dropwise addition, react at 60°C for 20-24h. After the reaction, filter with suction to remove the activated carbon, wash the filter cake with 25ml of DMF, and spin evaporate the obtained filtrate at 85°C to remove the solvent. Add 25ml of methanol to the concentrate after rotary evaporation, add 25ml of water dropwise under ice bath, continue to stir for 1h under ice bath after the dropwise addition, filter with suction, and dry the filter cake at 50°C for 16h to obtain khaki LA- 2: 3.4g (yield: 86.4%, liquid phase purity 98.1%, melting point 244.4~245.8°C)
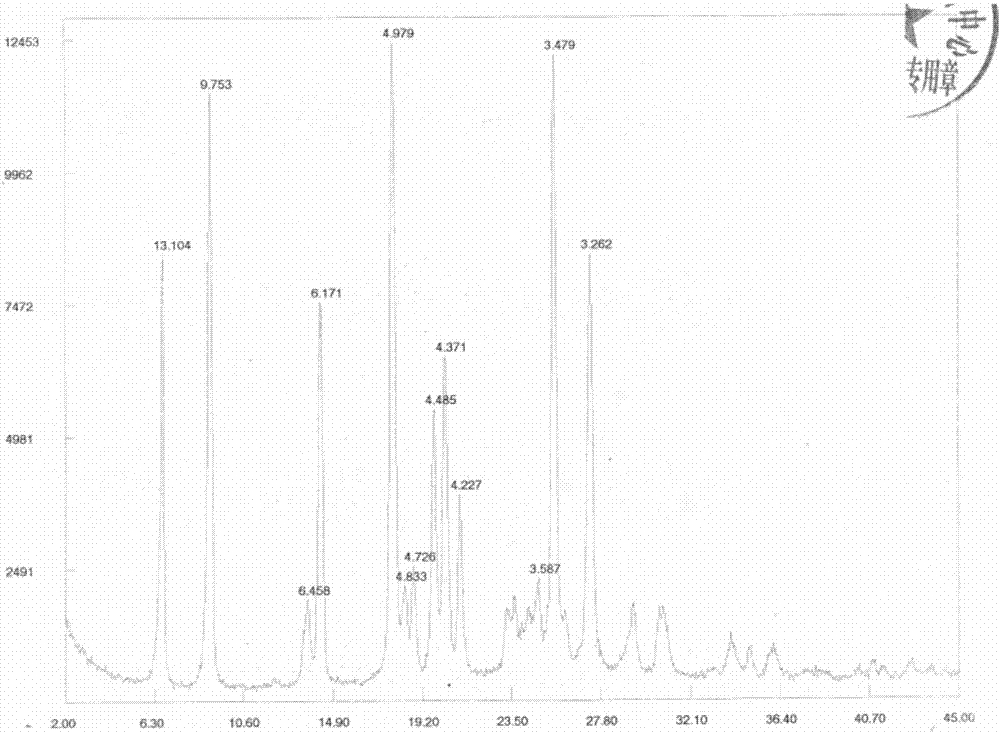
[0078] 1 H-NMR (DMSO-d 6):4.068(s, 2H), 5.782(s, 2H), 5.836(s, 4H), 7.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com