Method for preparing 5-(4-bromophenyl)-4,6-dichloropyrimidine
a technology of bromophenyl and pyrimidine, which is applied in the direction of physical/chemical process catalysts, metal/metal-oxide/metal-hydroxide catalysts, organic chemistry, etc., can solve the problems of increased production cost and waste of resources, and achieves easy separation, simple synthesis process and post-treatment steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
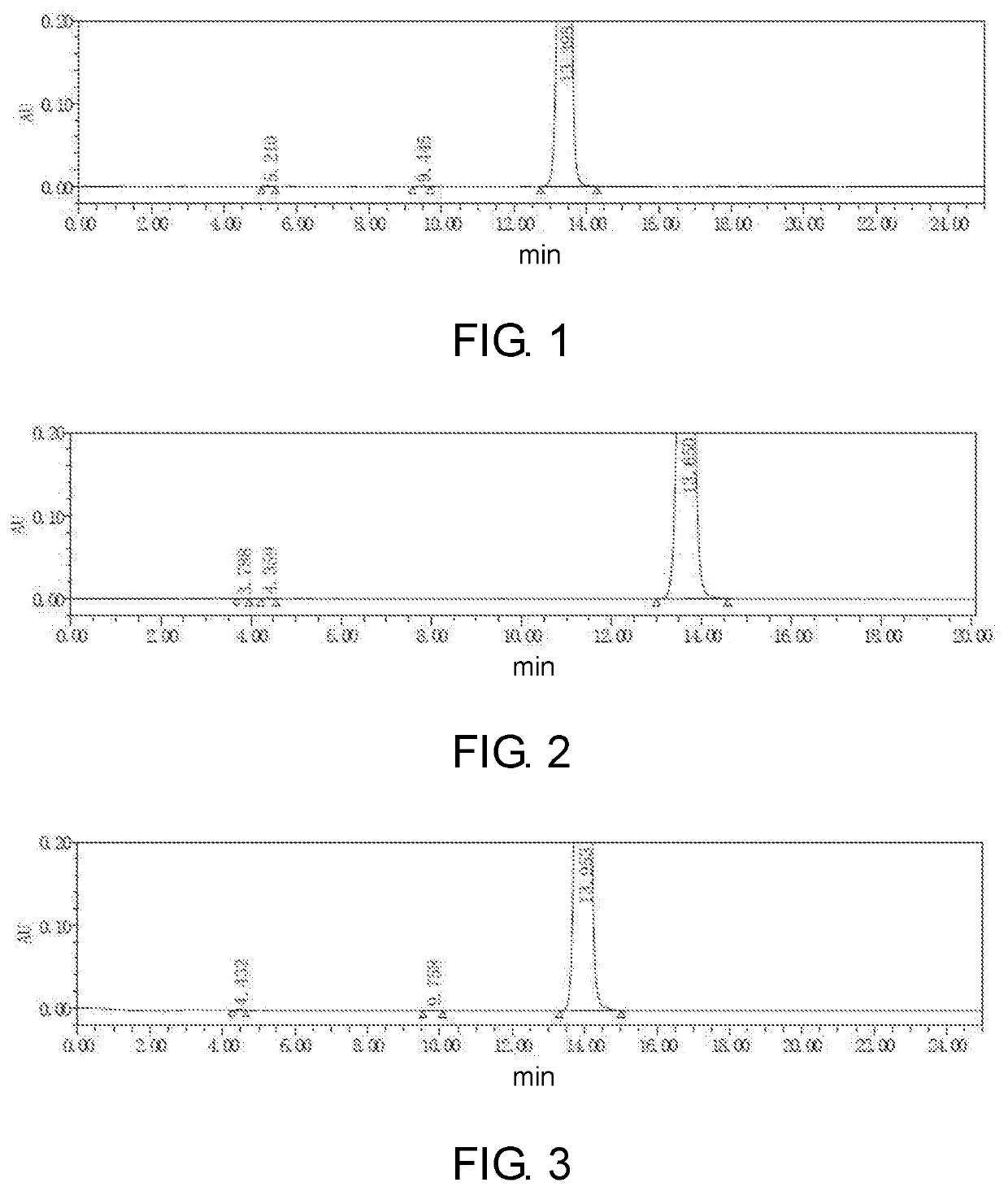
[0045]In this embodiment, a method for preparing 5-(4-bromophenyl)-4,6-dichloropyrimidine is provided, which comprises the following steps, specifically.
[0046]Step 1: To a 5.0 L reaction flask, 500 g of p-bromophenylacetic acid and 200 g of a solid acid catalyst were added. Then, 2.7 L of methanol was added, heated with stirring and reacted under reflux for 5 hours.
[0047]The solid acid catalyst may be a complex of iron oxide, zirconium oxide, titanium oxide, diatomaceous earth or silica gel with sulfate ions loaded thereon, and may be purchased from Qufu Shengquan Catalyst Application Technology Co., Ltd. The solid acid catalyst used in examples that follows was the same as that in this example, and thus will not be described there again. The reaction solution was cooled to 20° C. or below, the solid acid catalyst was recovered by filtering, and the resulting filtrate was distilled under reduced pressure to remove methanol. 2.0 L of toluene was added to dissolve the residue by stirr...
example 2
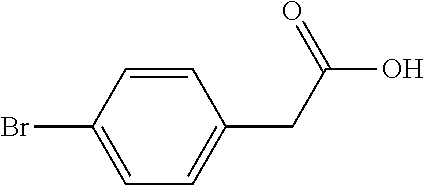
[0052]In this embodiment, a method for preparing 5-(4-bromophenyl)-4,6-dichloropyrimidine is provided, which comprises the following steps, specifically.
[0053]Step 1: To a 5.0 L reaction flask, 500 g of p-bromophenylacetic acid and 300 g of a solid acid catalyst were added. Then, 2.7 L of methanol was added, heated with stirring and reacted under reflux for 6 hours. The reaction solution was cooled to 30° C., the solid acid catalyst was recovered by filtering, and the resulting filtrate was distilled under reduced pressure to remove methanol. 2.0 L of toluene was added to dissolve the residue by stirring, and the toluene was washed with water, and concentrated under reduced pressure, to obtain 499.6 g of Intermediate 1 (yield 93.8%).
[0054]Step 2: 250 g of Intermediate 1 obtained in Step 1 was added to a 5 L four-neck flask, then 250 g of sodium methoxide and 1000 g of methanol were added, and mechanically stirred. Then, 375 g of dimethyl carbonate was added, the air in the reactor w...
example 3
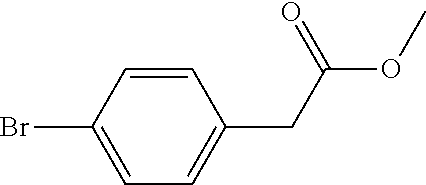
[0058]In this embodiment, a method for preparing 5-(4-bromophenyl)-4,6-dichloropyrimidine is provided, which comprises the following steps, specifically.
[0059]Step 1: To a 5.0 L reaction flask, 500 g of p-bromophenylacetic acid and 250 g of a solid acid catalyst were added. Then, 2.7 L of methanol was added, heated with stirring and reacted under reflux for 5.5 hours. The reaction solution was cooled to 25° C., the solid acid catalyst was recovered by filtering, and the resulting filtrate was distilled under reduced pressure to remove methanol. 2.0 L of toluene was added to dissolve the residue by stirring, and the toluene was washed with water, and concentrated under reduced pressure, to obtain 505.4 g of Intermediate 1 (yield 94.9%).
[0060]Step 2: 250 g of Intermediate 1 obtained in Step 1 was added to a 5 L four-neck flask, then 200 g of sodium methoxide and 1000 g of methanol were added, and mechanically stirred. Then, 312.5 g of dimethyl carbonate was added, the air in the react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com