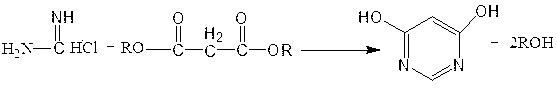Method for preparing 4,6-dihydroxypyrimidine
A technology of dihydroxypyrimidine and malonate, applied in the field of medicine, can solve problems such as the yield of only 78%, and achieve the effect of cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put 20g of formamidine hydrochloride, 40g of diethyl malonate, and 12g of sodium hydroxide into methanol, heat up and reflux for 9 hours, cool to 40°C after the reaction is completed, add hydrochloric acid dropwise to adjust the pH to about 7, recover methanol under reduced pressure, and recover After the end, add water to cool, stir and filter to obtain pale yellow crystals, and recrystallize with acetone to obtain 25.3 g of 4,6-dihydroxypyrimidine with a content of 98.9%.
Embodiment 2
[0022] Put 20g of formamidine hydrochloride, 60g of diethyl malonate, and 25g of potassium hydroxide into ethanol, raise the temperature and reflux for 4 hours, cool to 40°C after the reaction is completed, add hydrochloric acid dropwise to adjust the pH to about 7, recover the ethanol under reduced pressure, and recover After the end, add water to cool, stir and filter to obtain pale yellow crystals, and recrystallize with acetone to obtain 27.4 g of 4,6-dihydroxypyrimidine, with a content of 99.2%.
Embodiment 3
[0024] Put 20g of formamidine hydrochloride, 35g of dimethyl malonate, and 15g of sodium methoxide into methanol, heat up and reflux for 8 hours, cool to 40°C after the reaction is completed, add hydrochloric acid dropwise to adjust the pH to about 7, recover methanol under reduced pressure, and the recovery is complete Then add water to cool, stir and filter to obtain pale yellow crystals, and recrystallize with acetone to obtain 26.7g of 4,6-dihydroxypyrimidine, content: 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com