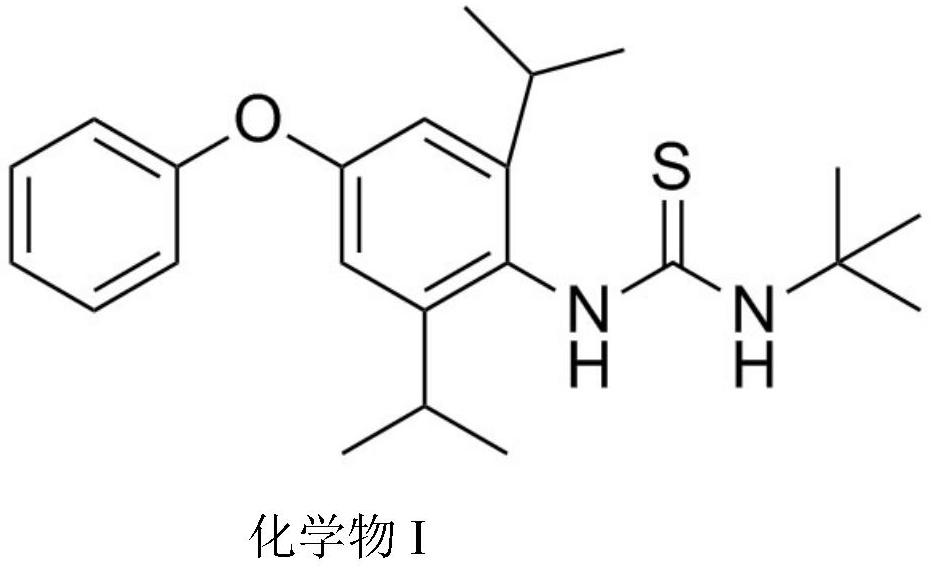
Synthesis method of diafenthiuron impurities A and B
A technology of diafenthiuron and impurities, applied in the field of medicinal chemistry, to achieve the effect of improving quality standards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
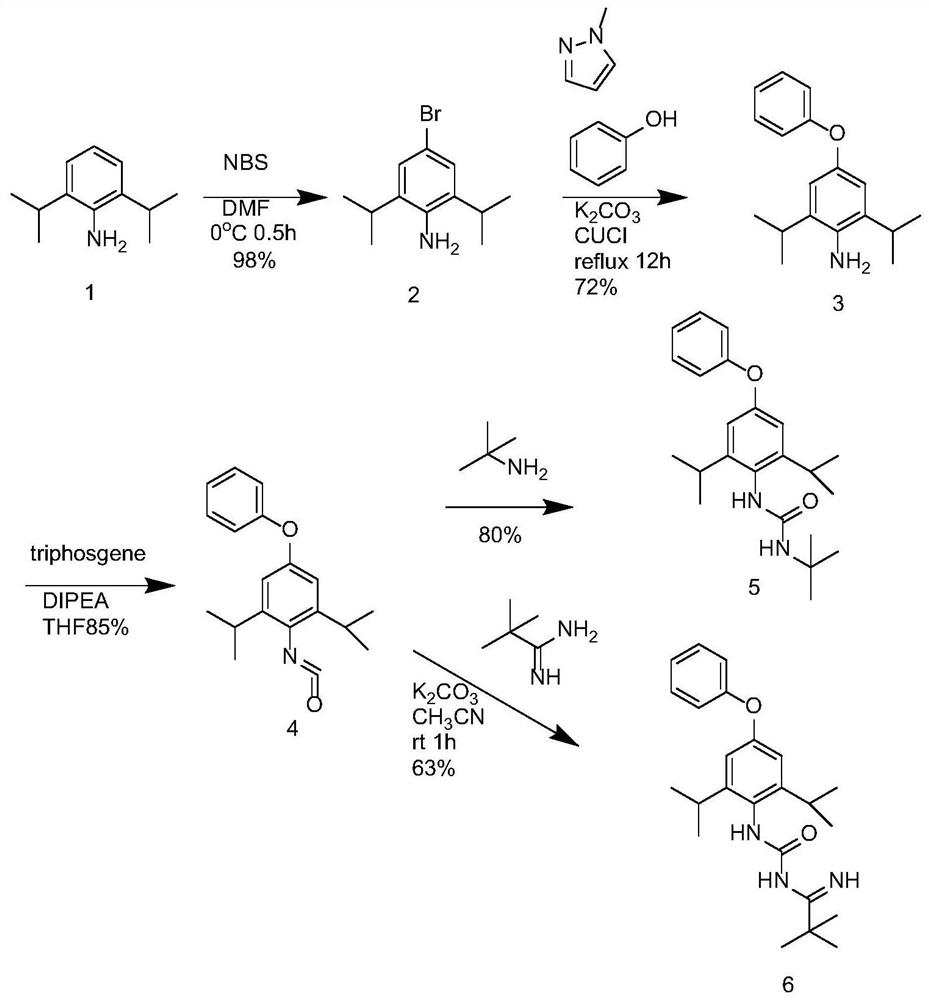
[0027] The invention provides the preparation method of diafenthiuron impurity A, such as figure 1 shown, including the following steps:
[0028] Step S1, dissolving 2,6-isopropylaniline in DMF to obtain the first reaction solution, adding the DMF solution of NBS dropwise, reacting for a period of time, adding water and ethyl acetate and mixing evenly, separating the liquids to obtain the first organic phase, The first organic phase was washed with water, dried and concentrated to obtain compound 2;
[0029] Step S2, dissolving the compound 2, potassium carbonate, phenol, cuprous chloride, and 1-methylimidazole in an organic solvent to obtain a second reaction solution, heating to reflux until the reaction is complete, adding water and ethyl acetate to mix evenly, Separation to obtain a second organic phase, which was washed with alkali, dried, concentrated, and column chromatographed to obtain compound 3;
[0030] Step S3, adding and dissolving the compound 3 and DIPEA in T...
Embodiment 1
[0038] This embodiment provides the preparation method of compound 2 (i.e. step S1):
[0039] Take 2,6-isopropylaniline (compound 1, 53g, 0.3mol), dissolve it in N,N-dimethylformamide (DMF, 500ml), lower the temperature to 0-5°C to obtain the first reaction solution, drop Add N-bromosuccinimide (NBS 63g, 0.36mol) in DMF (200ml) solution, after the dropwise addition is completed, react at 0-5°C for 0.5 hours, TLC detection confirms that the reaction is complete, add water and ethyl acetate and stir After 10 minutes, the liquid was separated, the organic phase was washed twice with water, dried and concentrated to obtain compound 2 (70 g, yield 98%);
[0040] The prepared compound 2 was detected by proton nuclear magnetic spectrum:
[0041] 1H NMR(300MHz,DMSO)δ6.93(s,2H),4.76(s,2H),2.93–3.04(m,2H), 1.10(d,J=6.9Hz,12H)
Embodiment 2
[0043] This embodiment provides the preparation method of compound 3 (i.e. step S2):
[0044]Take compound 2 (30g, 0.11mol), potassium carbonate (32.4g, 0.23mol), phenol (13.2g, 0.14mol), cuprous chloride (0.58mol), 1-methylimidazole (4.7ml) was dissolved in di The second reaction liquid was obtained in toluene (300ml), heated to reflux for 12 hours, added water and ethyl acetate after cooling, stirred for 10 minutes, separated, the organic phase was washed with potassium carbonate aqueous solution, dried over sodium sulfate, concentrated, and subjected to column chromatography Obtain compound 3 (23g, yield 72%)
[0045] The prepared compound 3 was detected by proton nuclear magnetic spectrum:
[0046] 1H NMR (300MHz,DMSO)7.21-7.23(m,2H),6.93-7.01(m,1H),6.79-6.86(m,2H),6.60(s,2H),4.50(s,2H),2.98- 3.09(m,2H), 1.11(d,J=6.9Hz,12H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com