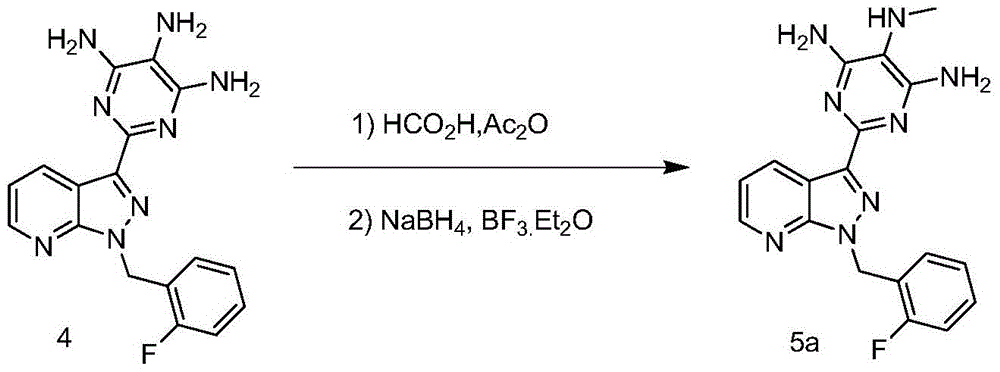A kind of synthetic method of riociguat
A synthetic method and compound technology, applied in the direction of organic chemistry, can solve the problems of troublesome operation, difficult purification, low total yield, etc., and achieve the effect of simple operation, mature process method and thorough reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment relates to a synthetic method of high-purity riociguat, comprising the following steps:
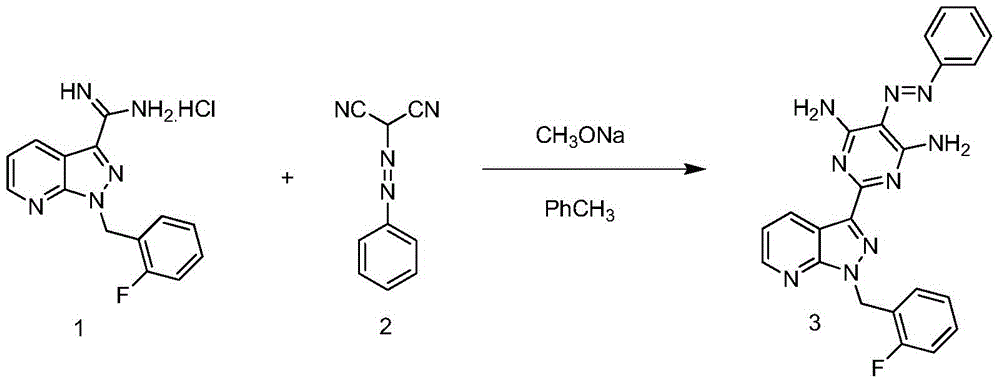
[0031] Step 1, in a 2L three-necked flask, sequentially add 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride (compound 1, 94.5g, 0.31 mol), 1.2L toluene, add sodium methoxide (17g, 0.31mol) and phenyl azomalononitrile (compound 2, 252.5g, 0.31mol) successively under stirring, heat to reflux at 120°C, TLC detects that the reaction is complete, cool to room temperature, suction filtration, the filter cake was washed once with toluene, and the solid was beaten with water, suction filtered, and dried to obtain intermediate compound 3 (129g, 0.29mol, yield 95%, content 99%), 1 H-NMR (400MHz, DMSO-d 6 ):δ=9.20(dd,J=1.5,8.1Hz,1H),8.66(dd,J=1.5,4.5Hz,1H),8.52(dd,brs,2H),8.02(d,J=7.2Hz, 2H),7.90(brs,2H),7.47~7.51(m,2H),7.34~7.43(m,3H),7.14~7.27(m,3H),5.85(s,2H);
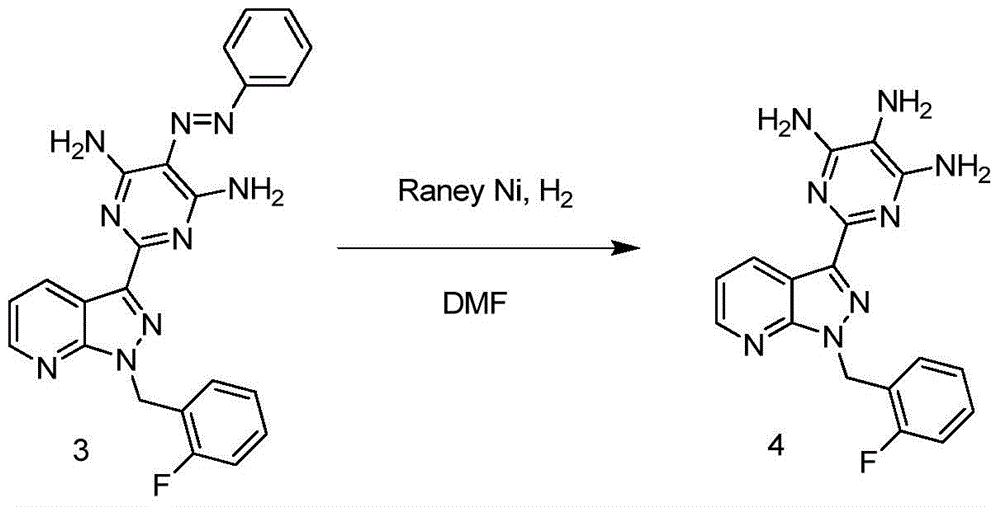
[0032] Step 2: Add the above-mentioned compound 3 (129 g, 0.29 mol) and 2.8 LDMF t...
Embodiment 2
[0036] This embodiment relates to a synthetic method of high-purity riociguat, comprising the following steps:
[0037] Step 1, in a 2L three-necked flask, sequentially add 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride (compound 1, 94.5g, 0.31 mol), 2L toluene, add sodium methoxide (25.5g, 0.46mol) and phenyl azomalononitrile (compound 2, 78.7g, 0.46mol) successively under stirring, heat to reflux at 115°C, TLC detects that the reaction is complete, cool After reaching room temperature, suction filtration, the filter cake was washed once with toluene, and the solid was beaten with water, suction filtered, and dried to obtain intermediate compound 3 (132 g, 0.30 mol, yield 98%, content 99%);
[0038] Step 2: Add the above-mentioned compound 3 (132g, 0.30mol) and 5LDMF into a 10L hydrogenation kettle in turn, add 20g of wet Raney nickel at room temperature, replace with nitrogen, then fill with hydrogen, press 3MPa, and control the temperature at 60...
Embodiment 3
[0042] This embodiment relates to a synthetic method of high-purity riociguat, comprising the following steps:
[0043] Step 1, in a 2L three-necked flask, sequentially add 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride (compound 1, 0.31mol), 2L of toluene, add sodium methoxide (0.39mol) and phenyl azomalononitrile (compound 2, 0.39mol) successively under stirring, heat to reflux at 110°C, TLC detects that the reaction is complete, cool to room temperature, filter with suction, and use for filter cake Wash once with toluene, beat the solid with water, suction filter, and dry to obtain intermediate compound 3 (132 g, 0.30 mol, yield 98%, content 99%);
[0044] Step 2: Add the above-mentioned compound 3 (132g, 0.30mol) and 5LDMF to a 10L hydrogenation kettle in turn, add 26g of wet Raney nickel at room temperature, replace with nitrogen, then fill with hydrogen, the pressure is 3.5MPa, and the temperature is controlled at 55°C React for 20 hours, TL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com