Method for synthesizing riociguat
A synthesis method and compound technology, applied in organic chemistry and other directions, can solve the problems of low total yield, difficult purification, troublesome operation, etc., and achieve the effects of simple operation, mature process method and thorough reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] This embodiment relates to a synthetic method of high-purity riociguat, comprising the following steps:
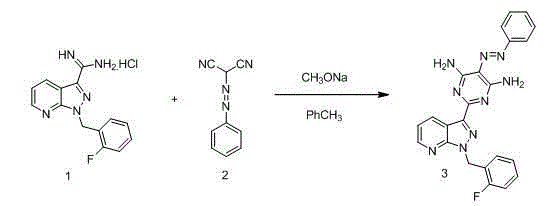
[0031] Step 1, in a 2 L three-necked flask, sequentially add 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride (compound 1, 94.5 g, 0.31mol), 1.2L toluene, add sodium methoxide (17g, 0.31mol) and phenyl azomalononitrile (compound 2, 252.5g, 0.31mol) successively under stirring, heat to reflux at 120°C, TLC detects that the reaction is complete, Cool to room temperature, filter with suction, wash the filter cake once with toluene, beat the solid with water, filter with suction, and dry to obtain intermediate compound 3 (129 g, 0.29 mol, yield 95%, content 99%), 1 H-NMR (400 MHz, DMSO-d 6 ): δ=9.20 (dd, J=1.5, 8.1Hz, 1H), 8.66 (dd, J=1.5, 4.5Hz, 1H), 8.52 (dd, brs, 2H), 8.02 (d, J=7.2Hz , 2 H), 7.90 (brs, 2H), 7.47~7.51 (m,2H), 7.34~7.43(m, 3H), 7.14~7.27(m, 3H), 5.85 (s, 2H);
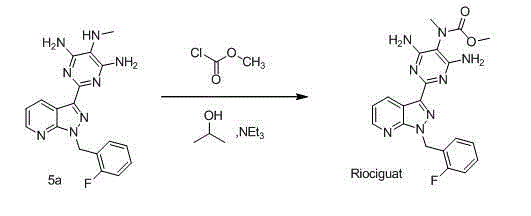
[0032] Step 2: Add the above-mentioned compound 3 (129 g, 0.2...
Embodiment 2
[0038] This embodiment relates to a synthetic method of high-purity riociguat, comprising the following steps:
[0039] Step 1, in a 2 L three-necked flask, sequentially add 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride (compound 1, 94.5 g, 0.31mol), 2L toluene, add sodium methoxide (25.5g, 0.46mol) and phenyl azomalononitrile (compound 2, 78.7g, 0.46mol) sequentially under stirring, heat to reflux at 115°C, TLC detects that the reaction is complete, Cool to room temperature, filter with suction, wash the filter cake once with toluene, beat the solid with water, filter with suction, and dry to obtain intermediate compound 3 (132 g, 0.30 mol, yield 98%, content 99%);
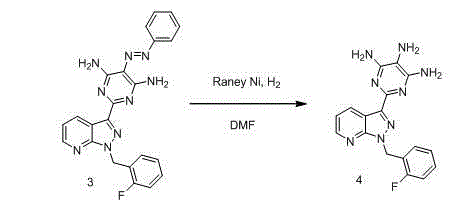
[0040] Step 2: Add the above-mentioned compound 3 (132 g, 0.30 mol) and 5 L DMF to a 10 L hydrogenation kettle in sequence, add 20 g of wet Raney nickel at room temperature, replace with nitrogen, and then fill with hydrogen, the pressure is 3 MPa, and the temperature is controlled ...
Embodiment 3
[0044] This embodiment relates to a synthetic method of high-purity riociguat, comprising the following steps:
[0045] Step 1, in a 2 L three-necked flask, sequentially add 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride (compound 1, 0.31mol) , 2L toluene, add sodium methoxide (0.39mol) and phenyl azomalononitrile (compound 2, 0.39mol) sequentially under stirring, heat to reflux at 110°C, TLC detects that the reaction is complete, cool to room temperature, filter with suction, filter cake Wash once with toluene, beat the solid with water, suction filter, and dry to obtain intermediate compound 3 (132 g, 0.30 mol, yield 98%, content 99%);
[0046] Step 2: Add the above-mentioned compound 3 (132 g, 0.30 mol) and 5 L DMF to a 10 L hydrogenation kettle in sequence, add 26 g of wet Raney nickel at room temperature, replace with nitrogen, and then fill with hydrogen, the pressure is 3.5 MPa, and the temperature Control the reaction at 55°C for 20 hours,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com