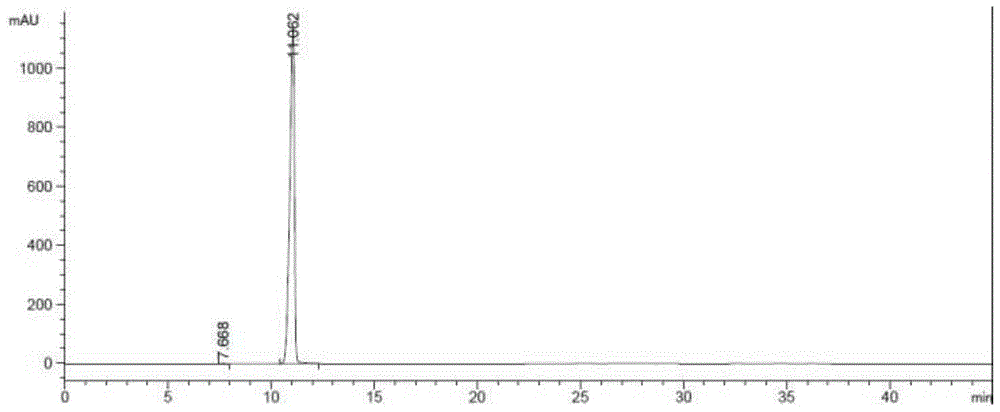
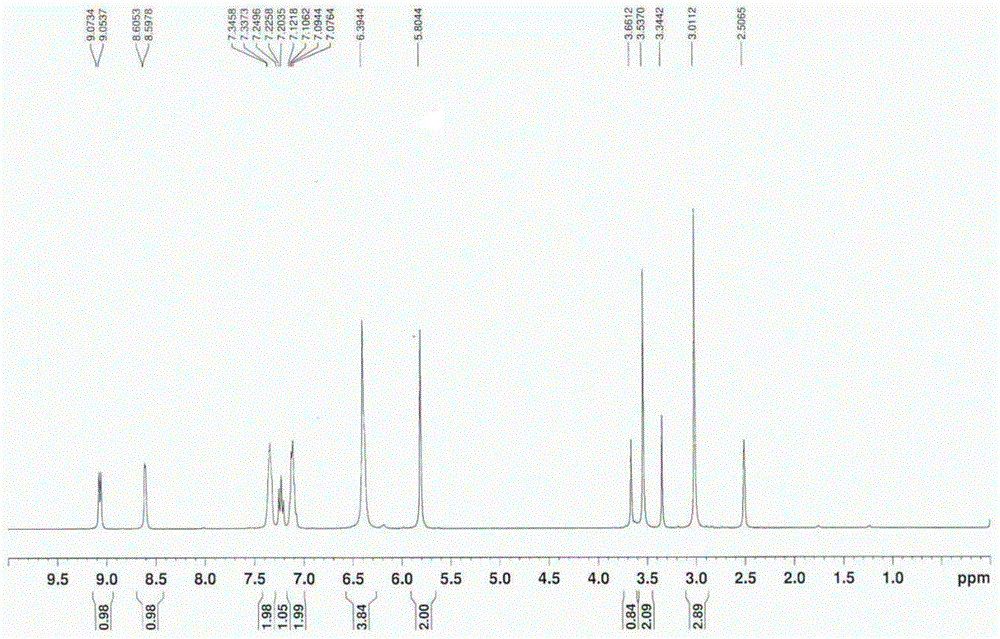
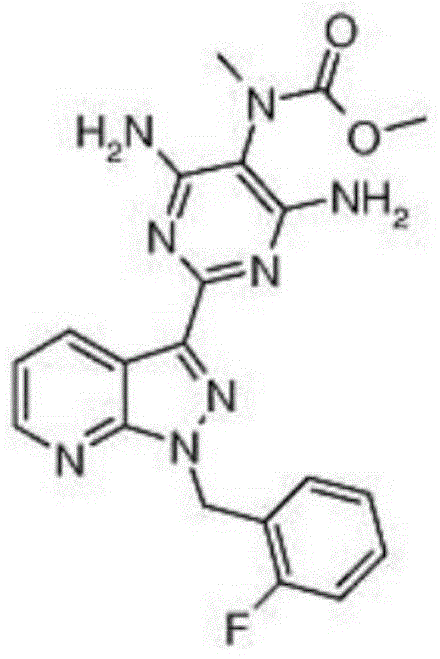
A kind of preparation method of riociguat
A technology of riociguat and compounds, applied in the field of preparation of riociguat, can solve the problems of difficult purification, troublesome operation, low total yield, etc., and achieve the goal of improving reaction yield, improving total yield, and high total yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation method of riociguat of the present invention, detailed steps are as follows:
[0050] a. First, add 31 g (0.1 mol) of 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, namely compound 1, into a 500 mL reaction flask, then Add 150mL methanol and 5.4g (0.1mol) sodium methoxide, heat to reflux; add 2-aminomalonamide dropwise at 65°C, that is, compound 2 solution (this solution is dissolved in 11.7g (0.1mol) 2-aminomalonamide prepared in 50 mL of methanol), the dropwise addition time was 30 min, and after the dropwise addition, the reflux reaction was carried out for 2 h to stop the reaction; the obtained reaction solution was cooled to room temperature, and then the solvent was evaporated under reduced pressure, and 100 mL of ethyl acetate was added to the obtained residue. Ester, a large amount of solids were generated and filtered, and the obtained filter cake was washed and filtered with 100 mL of water and 100 mL of ethyl acet...
Embodiment 2
[0055] The preparation method of riociguat of the present invention, detailed steps are as follows:
[0056] a. First, add 31 g (0.1 mol) of 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, namely compound 1, into a 500 mL reaction flask, then Add 150 mL of methanol and 5.94 g (0.11 mol) of sodium methoxide, heat to reflux; add 2-aminomalonamide dropwise at 60°C, that is, compound 2 solution (this solution is dissolved in 10.5 g (0.09 mol) of 2-aminomalonamide. It was prepared in 50 mL of methanol), the dropwise addition time was 30 min, and the reflux reaction was carried out for 2 h after the dropwise addition to stop the reaction; the obtained reaction solution was cooled to room temperature, the solvent was evaporated under reduced pressure, and 100 mL of ethyl acetate was added to the obtained residue. , a large amount of solids were generated and filtered, and the obtained filter cake was washed and filtered with 100 mL of water and 100 mL of ...
Embodiment 3
[0061] The preparation method of riociguat of the present invention, detailed steps are as follows:
[0062] a. First, add 31 g (0.1 mol) of 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, namely compound 1, into a 500 mL reaction flask, then Add 150 mL methanol and 8.1 g (0.15 mol) sodium methoxide, heat to reflux; add 2-aminomalonamide, that is, compound 2 solution, dropwise at 65°C (this solution is dissolved in 12.9 g (0.11 mol) 2-aminomalonamide It was prepared in 50 mL of methanol), the dropwise addition time was 30 min, and the reflux reaction was carried out for 2 h after the dropwise addition to stop the reaction; the obtained reaction solution was cooled to room temperature, the solvent was evaporated under reduced pressure, and 100 mL of ethyl acetate was added to the obtained residue. , a large amount of solids were generated and filtered, and the obtained filter cake was washed and filtered with 100 mL of water and 100 mL of ethyl acet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com