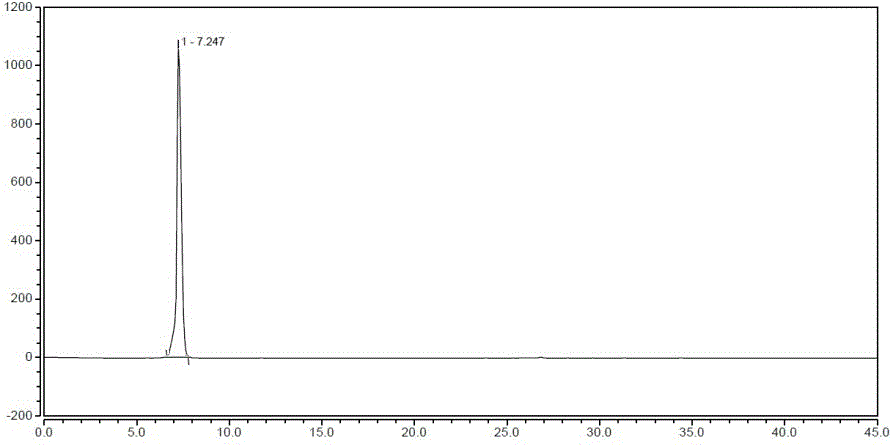
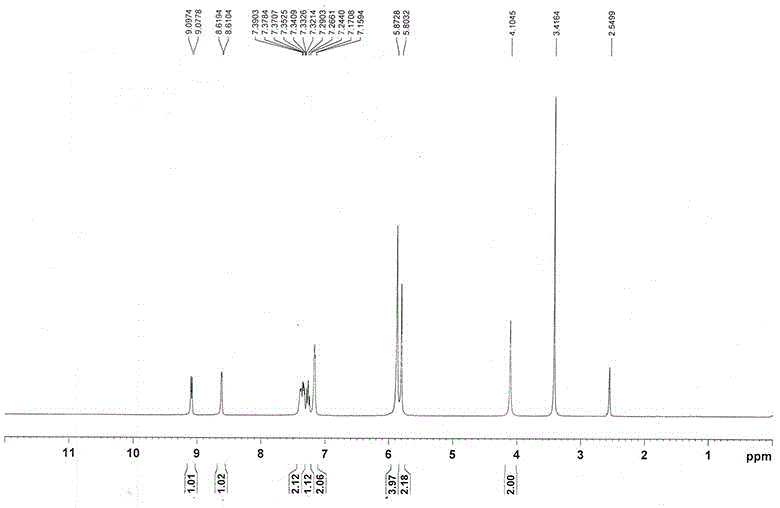
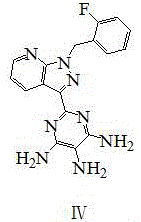
Method for compounding riociguat intermediate
A synthesis method and technology for intermediates, which are applied in the field of synthesis of riociguat intermediates, can solve the problems of high risk factor, low content, difficult purification and the like, and achieve the requirements of reducing equipment, reducing production costs and improving reaction yields. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] a. Add 50.0g (0.16mol) 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, 27.2g (0.16mol) to a 500ml reaction flask mol) phenylpropazomalononitrile, 8.6g (0.16mol) sodium methoxide and 350ml DMF, stirred and reacted at 110°C for 12h, cooled to 25°C, filtered to obtain a solid, stirred and washed with 250ml of ethanol for 10min, and filtered to obtain a solid, Repeat the above washing steps twice to obtain a solid, which was dried in a drying oven at 85°C for 5 hours to obtain 60.7 g of compound III with a yield of 84.6%;
[0035] b. Add 60.7g (0.138mol) of compound III, 7.4g (0.138mol) of ammonium chloride and 730ml of ethanol / water into a 1L reaction flask, add 5.8g (0.1mol) of iron powder under stirring, and stir the reaction at 79°C After 8 hours, cool down to 25°C, filter to obtain the filtrate, distill under reduced pressure (vacuum degree: 0.090MPa, temperature: 65°C) to obtain the residue, add 120ml of water to the residue and stir for 3...
Embodiment 2
[0037] a. Add 50.0g (0.16mol) 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, 29.9g (0.176 mol) phenylpropazomalononitrile, 21.8g (0.32mol) sodium ethoxide and 500ml DMF, stirred and reacted at 120°C for 15h, cooled to 20°C, filtered to obtain a solid, stirred and washed with 250ml of ethanol for 10min, and filtered to obtain a solid, The above washing steps were repeated twice to obtain a solid, which was dried in a drying oven at 80°C for 5 hours to obtain 57.4 g of compound III with a yield of 80.0%;
[0038] b. Add 57.4g (0.13mol) of compound III, 8.3g (0.156mol) of ammonium chloride and 860ml of ethanol / water into a 1L reaction flask, add 6.6g (0.117mol) of iron powder under stirring, and stir the reaction at 79°C After 10 hours, cool down to 30°C, filter to obtain the filtrate, distill under reduced pressure (vacuum degree: 0.090MPa, temperature: 70°C) to obtain the residue, add 120ml of water to the residue and stir for 30min, filter to obta...
Embodiment 3
[0040]a. Add 50.0g (0.16mol) 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, 33.4g (0.196 mol) phenylpropazomalononitrile, 5.9g (0.245mol) sodium hydride and 250ml DMF, stirred and reacted at 115°C for 10h, cooled to 30°C, filtered to obtain a solid, stirred and washed with 250ml of ethanol for 10min, and filtered to obtain a solid, The above washing steps were repeated twice to obtain a solid, which was dried in a drying oven at 90°C for 5 hours to obtain 59.8 g of compound III with a yield of 83.4%;
[0041] b. Add 59.8g (0.136mol) of compound III, 10.9g (0.2mol) of ammonium chloride and 598ml of medicinal ethanol / water into a 1L reaction flask, add 7.6g (0.136mol) of iron powder under stirring, and store at 79°C After stirring for 6 hours, cool down to 20°C, filter to obtain the filtrate, distill under reduced pressure (vacuum degree: 0.090MPa, temperature: 60°C) to obtain the residue, add 120ml of water to the residue, stir for 30min, and filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com