Preparation method of 2,4-di-substituted-1,3,5-triazine
A disubstituted, formamidine hydrochloride technology, applied in the direction of organic chemistry, can solve the problems of unfriendly demethylation by-products, limited range of substrates, and insufficient mild conditions, and achieve low-cost, low-toxic metal residues and environmental protection. Friendly, no metal residue effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
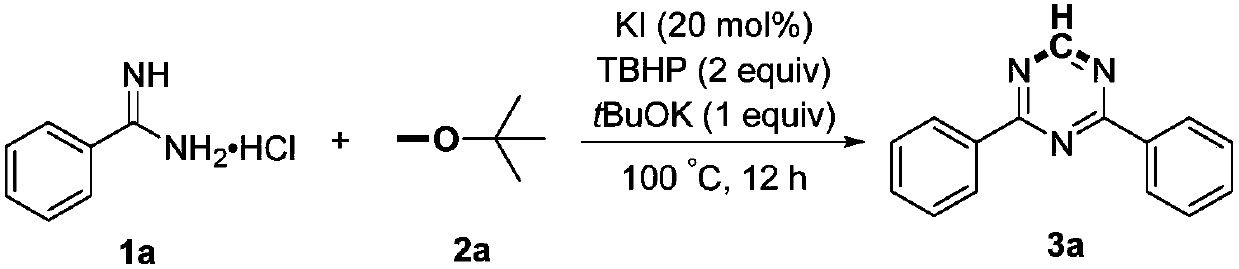
[0014]
[0015] Add 1a (4mmol, 624mg), potassium iodide (0.8mmol, 133mg), potassium tert-butoxide (4mmol, 449mg), tert-butyl hydroperoxide (8mmol, 70% aqueous solution, 1.2mL) and methyl tert-butyl to the reaction flask base ether (10 mL), and then heated at 100° C. for 12 hours. After the reaction is completed, it is first quenched with saturated sodium thiosulfate solution, then extracted with ethyl acetate, the organic phases are combined, dried with anhydrous sodium sulfate, concentrated, and then subjected to column chromatography with a mixed solvent of petroleum ether and ethyl acetate to obtain Product 3a, yield 85%. White solid, Mp: 74-75℃; 1 H NMR (600MHz, CDCl 3 ):δ9.25(s,1H),8.65-8.63(m,4H),7.62-7.58(m,2H),7.56-7.53(m,4H); 13 C NMR (150MHz, CDCl 3 ): δ171.3, 166.7, 135.5, 132.8, 128.9, 128.7.
Embodiment 2
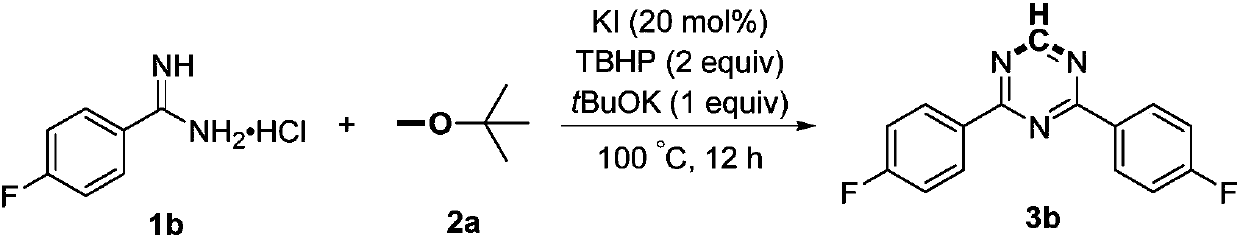
[0017]
[0018] Add 1b (4mmol, 698mg), potassium iodide (0.8mmol, 133mg), potassium tert-butoxide (4mmol, 449mg), tert-butyl hydroperoxide (8mmol, 70% aqueous solution, 1.2mL) and methyl tert-butyl to the reaction flask base ether (10 mL), and then heated at 100° C. for 18 hours. After the reaction is completed, it is first quenched with saturated sodium thiosulfate solution, then extracted with ethyl acetate, the organic phases are combined, dried with anhydrous sodium sulfate, concentrated, and then subjected to column chromatography with a mixed solvent of petroleum ether and ethyl acetate to obtain Product 3b, yield 69%. White solid, Mp: 155-156°C; 1 H NMR (600MHz, CDCl 3 ):δ9.20(s,1H),8.67-8.62(m,4H),7.25-7.20(m,4H); 13 C NMR (150MHz, CDCl 3 ): δ 170.3, 166.6, 166.0 (d, J=252.6Hz), 131.6 (d, J=3.0Hz), 131.3 (d, J=9.4Hz), 115.9 (d, J=21.5Hz).
Embodiment 3
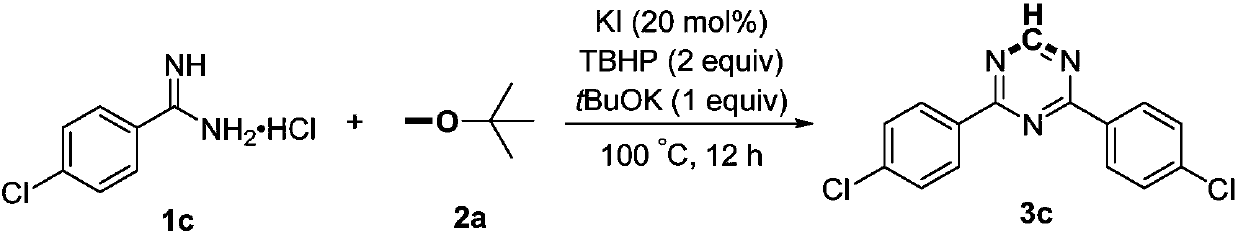
[0020]
[0021] Add 1c (4mmol, 764mg), potassium iodide (0.8mmol, 133mg), potassium tert-butoxide (4mmol, 449mg), tert-butyl hydroperoxide (8mmol, 70% aqueous solution, 1.2mL) and methyl tert-butyl to the reaction flask base ether (10 mL), and then heated at 100° C. for 12 hours. After the reaction is completed, it is first quenched with saturated sodium thiosulfate solution, then extracted with ethyl acetate, the organic phases are combined, dried with anhydrous sodium sulfate, concentrated, and then subjected to column chromatography with a mixed solvent of petroleum ether and ethyl acetate to obtain Product 3c, yield 81%. White solid, Mp: 191-192°C; 1 H NMR (600MHz, CDCl 3 ):δ9.23(s,1H),8.58-8.55(m,4H),7.53-7.50(m,4H); 13 C NMR (150MHz, CDCl 3 ): δ170.5, 166.7, 139.4, 133.8, 130.2, 129.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com