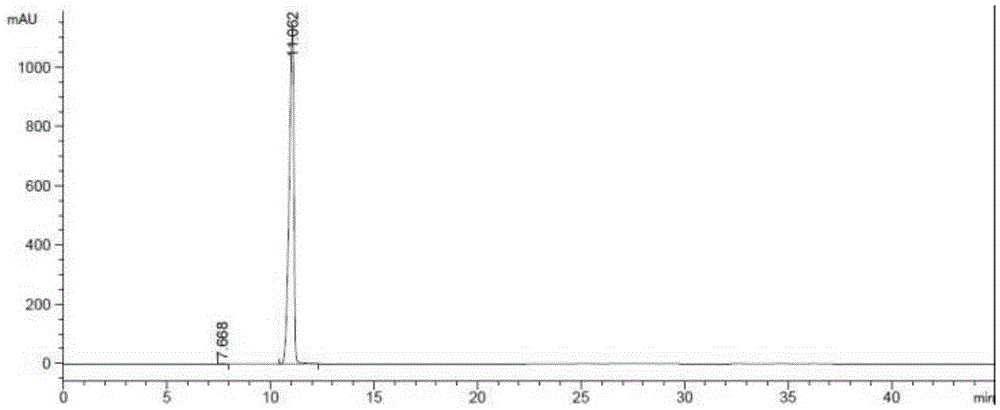
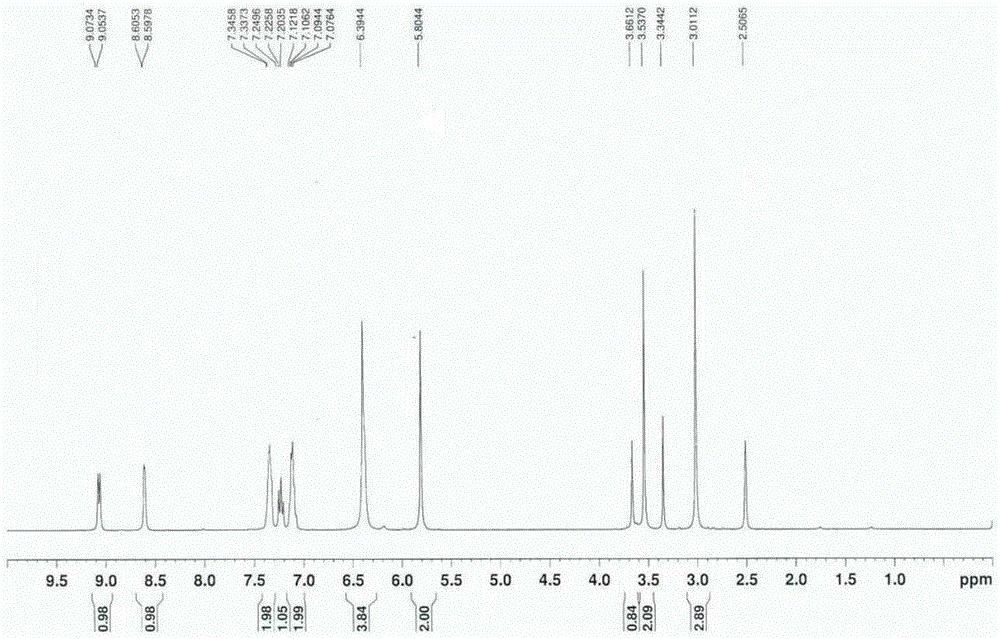
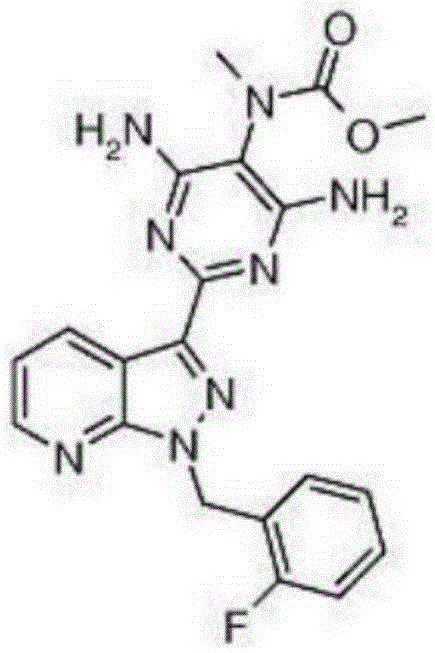
Preparation method of riociguat
A technology for riociguat and compound, applied in the field of preparation of riociguat, can solve the problems of low total yield, troublesome operation, difficult purification and the like, and achieves high total yield, improved total yield and improved reaction yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation method of riociguat of the present invention, detailed steps are as follows:
[0045] a. First, add 31g (0.1mol) of 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, compound 1, into a 500mL reaction flask, and then Add 150mL methanol and 5.4g (0.1mol) sodium methoxide, and heat to reflux; add dropwise 2-aminomalonamide, that is, compound 2 solution (the solution is dissolved in 11.7g (0.1mol) 2-aminomalonamide) at 65°C prepared in 50mL of methanol), the dropwise addition time is 30min, after the dropwise addition, reflux reaction is carried out for 2h, and the reaction is stopped; Esters, a large amount of solids were formed, filtered, the resulting filter cake was washed with 100mL of water and 100mL of ethyl acetate, filtered, and the obtained solids were dried at 65°C and a vacuum of 0.09MPa for 5h to obtain 28.4g of 2-[1-(2-fluoro Benzyl)-1H-pyrazolo[3,4-b]pyridin-3-yl]pyrimidine-4,5,6-triamine is compound 3 (yield 80%); ...
Embodiment 2
[0050] The preparation method of riociguat of the present invention, detailed steps are as follows:
[0051] a. First, add 31g (0.1mol) of 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, compound 1, into a 500mL reaction flask, and then Add 150mL of methanol and 5.94g (0.11mol) of sodium methoxide, and heat to reflux; add dropwise 2-aminomalonamide, that is, compound 2 solution at 60°C (the solution is dissolved in 10.5g (0.09mol) of 2-aminomalonamide prepared in 50mL of methanol), the dropwise addition time is 30min, after the dropwise addition, reflux reaction is carried out for 2h, and the reaction is stopped; after the obtained reaction solution is lowered to room temperature, the solvent is evaporated under reduced pressure, and 100mL of ethyl acetate is added to the obtained residue , a large amount of solids were formed and filtered. The resulting filter cake was washed with 100 mL of water and 100 mL of ethyl acetate and filtered. The obtai...
Embodiment 3
[0056] The preparation method of riociguat of the present invention, detailed steps are as follows:
[0057] a. First, add 31g (0.1mol) of 1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine-3-carboxamidine hydrochloride, compound 1, into a 500mL reaction flask, and then Add 150mL of methanol and 8.1g (0.15mol) of sodium methoxide, and heat to reflux; dropwise add 2-aminomalonamide, that is, compound 2 solution at 65°C (the solution is dissolved in 12.9g (0.11mol) of 2-aminomalonamide prepared in 50mL of methanol), the dropwise addition time is 30min, after the dropwise addition, reflux reaction is carried out for 2h, and the reaction is stopped; after the obtained reaction solution is lowered to room temperature, the solvent is evaporated under reduced pressure, and 100mL of ethyl acetate is added to the obtained residue , a large amount of solids were formed and filtered, and the obtained filter cake was washed with 100 mL of water and 100 mL of ethyl acetate, and filtered, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com