Preparation method of riociguat intermediate
A technology for riociguat and intermediates, applied in the field of pharmaceutical synthesis, can solve the problems of difficult post-processing, increased impurities, complicated preparation process, etc., to avoid inflammable, explosive and toxic reagents, reduce equipment requirements, and simplify process operations. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
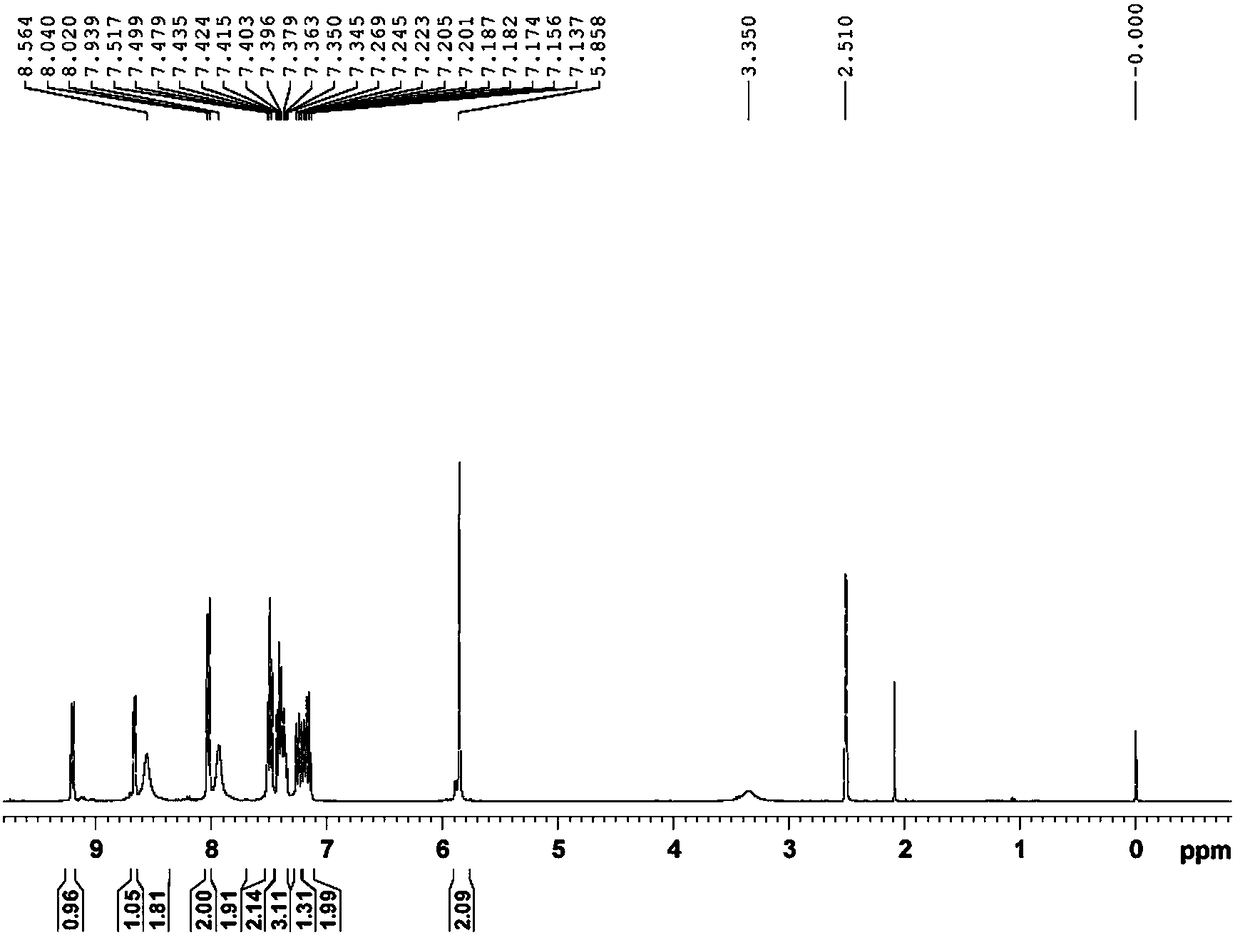
[0047] The preparation of embodiment 1 compound III
[0048] Add 1.5L of N,N-dimethylformamide, compound I (250g, 0.82mol), compound II (140g, 0.82mol), 30% sodium methoxide solution (150ml, 0.83mol), and reaction solution into a 2L reaction flask in sequence Raise the temperature to 105-115°C, and keep the reaction for 15-16 hours. The reaction solution was added into 10 L of purified water, and stirred at room temperature for 4-5 hours. After filtering, the filter cake was placed in a blast drying oven at a controlled temperature of 50-60° C. and dried for 23-24 hours to obtain 356 g (0.81 mol) of compound III as a reddish-brown solid, with a molar yield of 98.8% and an HPLC purity of 99.5%.
Embodiment 2
[0049] The preparation of embodiment two compound III
[0050] Add N,N-dimethylacetamide 1.5L, compound I (250g, 0.82mol), compound II (140g, 0.82mol), 50% sodium ethoxide solution (115ml, 0.83mol), reaction solution into 2L reaction flask successively Raise the temperature to 110-115°C, and keep the reaction for 14-16 hours. The reaction solution was added into 10 L of purified water, and stirred at room temperature for 4-5 hours. After filtering, the filter cake was placed in a blast drying oven at a controlled temperature of 50-60° C. and dried for 23-24 hours to obtain 351 g (0.80 mol) of compound III as a reddish-brown solid, with a molar yield of 97.6% and an HPLC purity of 99.2%.
Embodiment 3
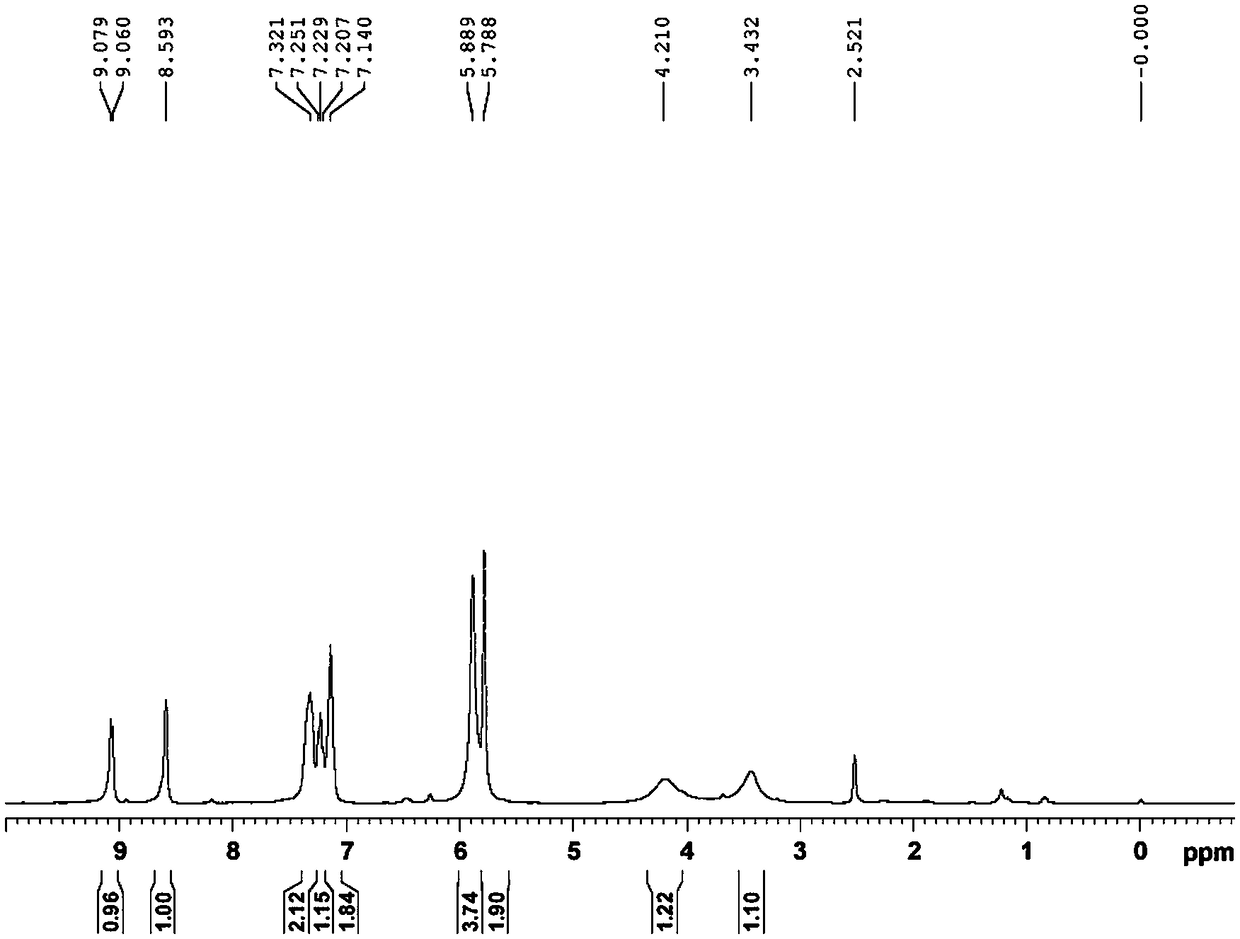
[0051] The preparation of embodiment three compound IV
[0052] Add 750ml of absolute ethanol, compound III (15.0g, 34mmol), 85% hydrazine hydrate solution (80ml, 1.36mol) to the 1L reaction flask in turn, add 7.5g of 10% palladium carbon under stirring, heat to 70-80°C, keep warm React at 70-80°C for 12 hours. The reaction liquid was filtered, and the filtrate was concentrated to dryness under reduced pressure, and 100 ml of absolute ethanol was added and stirred for 2 hours. After filtration, the filter cake was air-dried at 45-55° C. for 7-8 hours to obtain 9.95 g (28.4 mmol) of Compound IV as a white solid, with a molar yield of 83.5%, an HPLC purity of 99.4%, and a heavy metal content of <20 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com