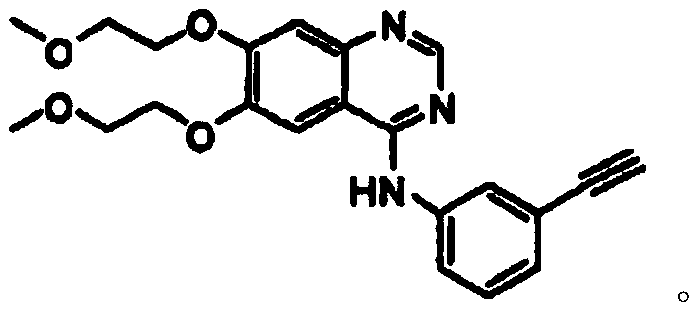
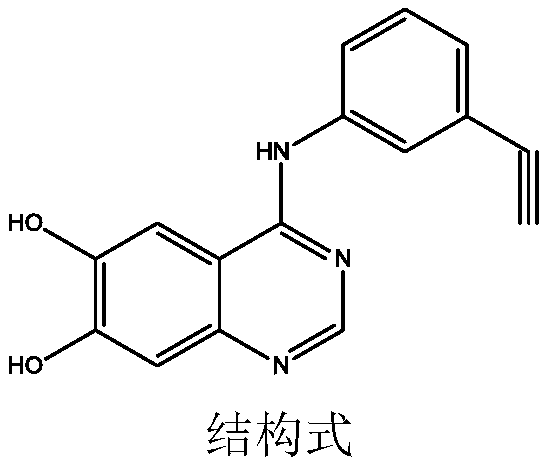
Synthetic method of erlotinib intermediate
A synthetic method and intermediate technology, applied in the field of drug synthesis, can solve problems such as research, achieve the effects of short reaction time, high reaction yield, and improved drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
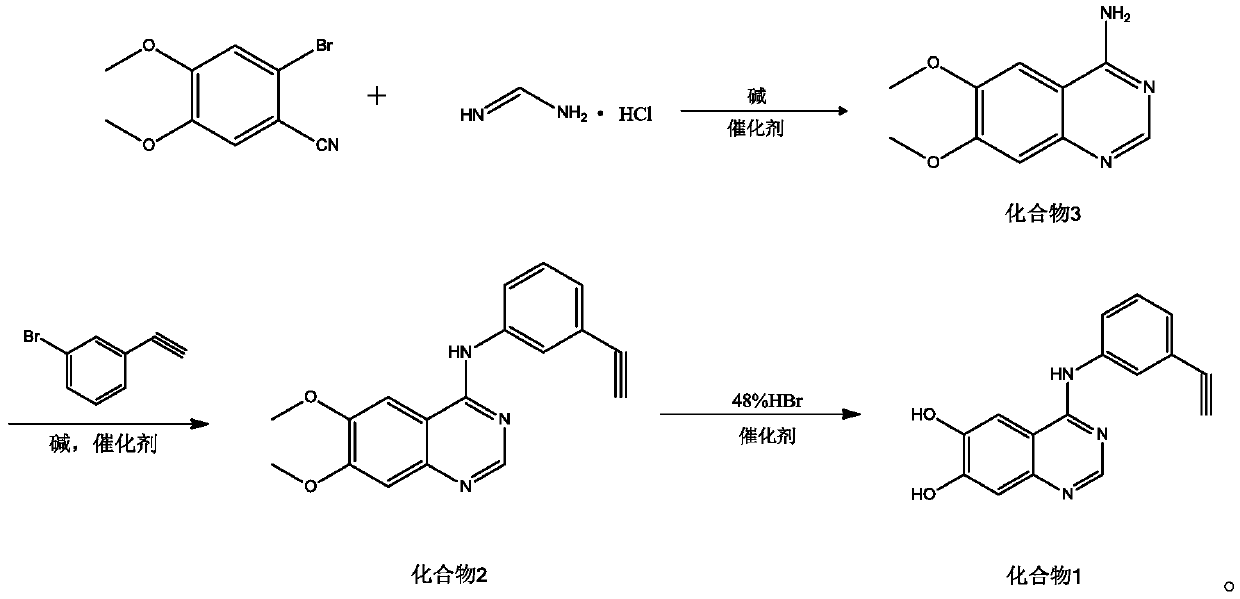
[0029] Embodiment 1: the synthesis of compound 3
[0030] in N 2 Under protection, 2-bromo-4,5-dimethoxybenzonitrile (50mmol), formamidine hydrochloride (55mmol), N,N'-dimethylethylenediamine (5mmol), K 2 CO 3 (100mmol) was dissolved in 150mL 1,4-dioxane, stirred for 10min, then CuI (2mmol) was added, heated to reflux for 2.5h, after the reaction was completed, cooled to room temperature, filtered, the filter cake was washed, and the filtrate and washing liquid were combined , The solvent was distilled off under reduced pressure, and the crude product was recrystallized from methanol, filtered and dried to obtain 9.303g of compound 3 with a yield of 90.60% and a purity of 99.92%.
Embodiment 2
[0031] Embodiment 2: the synthesis of compound 3
[0032] in N 2 Under protection, 2-bromo-4,5-dimethoxybenzonitrile (50mmol), formamidine hydrochloride (50mmol), N,N'-dimethylethylenediamine (0.5mmol), K 2 CO 3 (75mmol) was dissolved in 150mL 1,4-dioxane, stirred for 10min, then added CuI (1mmol), heated to reflux for 2.5h, after the reaction was completed, cooled to room temperature, filtered, washed the filter cake, and combined the filtrate and washing liquid , The solvent was distilled off under reduced pressure, and the crude product was recrystallized from methanol, filtered and dried to obtain 8.477g of compound 3 with a yield of 80.49% and a purity of 97.42%.
Embodiment 3
[0033] Embodiment 3: the synthesis of compound 3
[0034]in N 2 Under protection, 2-bromo-4,5-dimethoxybenzonitrile (50mmol), formamidine hydrochloride (60mmol), N,N'-dimethylethylenediamine (10mmol), K 2 CO 3 (125mmol) was dissolved in 150mL 1,4-dioxane, stirred for 10min, then added CuI (5mmol), heated to reflux for 2.5h, after the reaction was completed, cooled to room temperature, filtered, washed the filter cake, and combined the filtrate and washing liquid , the solvent was distilled off under reduced pressure, and the crude product was recrystallized from methanol, filtered and dried to obtain 8.806g of compound 3 with a yield of 84.34% and a purity of 98.27%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com