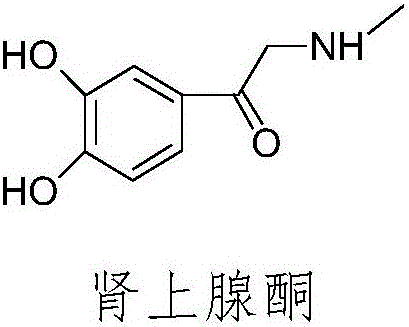
Preparation method of adrenalone hydrochloride
A technology of epinephrine hydrochloride and epinephrine, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The present embodiment relates to a kind of preparation method of epinephrine hydrochloride, comprises the steps:
[0033] 1) Add 10g (0.054mol) of chloroacetylcatechol, 39g of ethanol solution of methylamine (containing 30% methylamine, 0.378mol) and 0.3g (0.0008mol) of tetrabutylammonium iodide into a 250ml three-necked reaction flask Stir at 30°C to make the material uniform. Nitrogen was introduced to replace the air in the reaction flask 4 times, and nitrogen was passed through the whole process, and reacted at a temperature of 30° C. for 6 hours until the reaction solution was gray-green. Stop the reaction, filter to obtain a gray-green solid, wash the gray-green solid with absolute ethanol and drain it to obtain a gray-green solid tidal product of epinephrine;
[0034] 2) Add the gray-green solid tidal product of epinephrine into a mixed solution of ethanol and concentrated hydrochloric acid (30ml of ethanol, 9ml of concentrated hydrochloric acid) for salt forma...
Embodiment 2
[0037] The present embodiment relates to a kind of preparation method of epinephrine hydrochloride, comprises the steps:
[0038] 1) Add 10g (0.054mol) of chloroacetylcatechol, 61.3g of ethanol solution of methylamine (containing 30% methylamine, 0.594mol) and 0.3g (0.0008mol) of tetrabutylammonium iodide to 250ml three-port reaction In the bottle, stir at 30°C to make the material uniform. Nitrogen was introduced to replace the air in the reaction bottle for 4 times, and then the reaction flask was protected by nitrogen, and reacted at a temperature of 30° C. for 4 hours. until the reaction solution is gray-green. Stop the reaction, filter to obtain a gray-green solid, wash the gray-green solid with absolute ethanol and drain it to obtain epinephrine;
[0039] 2) Add the gray-green solid tidal product of epinephrine into a mixed solution of ethanol and concentrated hydrochloric acid (30ml ethanol, 9ml concentrated hydrochloric acid) for salt formation reaction, stir, and ad...
Embodiment 3
[0042] The present embodiment relates to a kind of preparation method of epinephrine hydrochloride, comprises the steps:
[0043] 1) Add 10g (0.054mol) of chloroacetylcatechol, 39g of ethanol solution of methylamine (containing 30% methylamine, 0.378mol) and 0.6g (0.0012mol) of tetrabutylammonium iodide into a 250ml three-necked reaction flask Stir at 40°C to make the material uniform. The air in the reaction flask was replaced by nitrogen gas for 4 times, and then protected by nitrogen gas, and the reaction was carried out at 30° C. for 5 h. until the reaction solution is gray-green. Stop the reaction, filter to obtain a gray-green solid, wash the gray-green solid with absolute ethanol and drain it to obtain epinephrine;
[0044] 2) Add the gray-green solid tidal product of epinephrine into a mixed solution of ethanol and concentrated hydrochloric acid (30ml ethanol, 9ml concentrated hydrochloric acid) for salt formation reaction, stir, and adjust the pH of the reaction sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com