Method for synthesizing 3-indole selenide alcohol organic compound
A technology for organic compounds and synthesis methods, applied in the field of organic compound synthesis, can solve the problems of metal catalysts that do not conform to environmental protection, unpleasant odor, etc., and achieves the effects of high atom utilization, efficient reaction, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
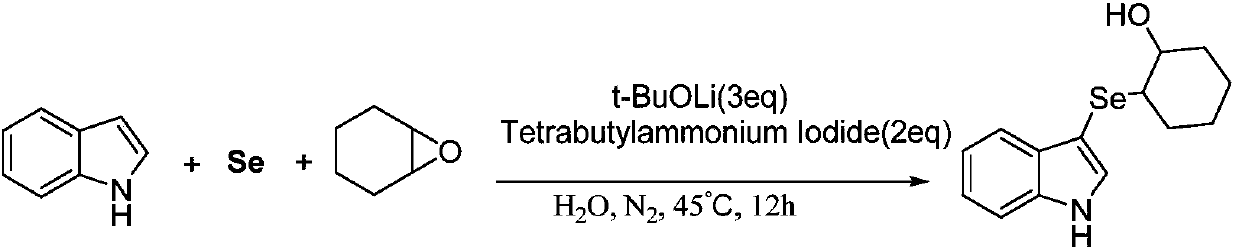
[0024] Synthesis of 2-(3-indoleselenyl)-1-cyclohexyl alcohol:
[0025]
[0026] At room temperature, add a stir bar, selenium powder (0.8mmol), lithium tert-butoxide (1.2mmol), phase transfer catalyst tetrabutylammonium iodide (0.8mmol), indole (0.4mmol) into a 25mL Schlenk tube, and then Vacuumize, fill with nitrogen, back and forth vacuum-fill nitrogen three times, add cyclohexene oxide (0.8mmol) and deionized water (2mL) under the protection of nitrogen, then stir at room temperature for 5min, and finally move to 45°C for heating React in the tank, use TLC (or GC-MS) tracking detection during the reaction, after detection, the reaction is complete after 12 hours.
[0027] After the reaction is completed and the reaction mixture is cooled, the reaction mixture is first diluted with 15 mL of water, then extracted three times with 45 mL of ethyl acetate, and the organic phase is collected three times, then dried with anhydrous sodium sulfate, filtered, concentrated under re...
Embodiment 2
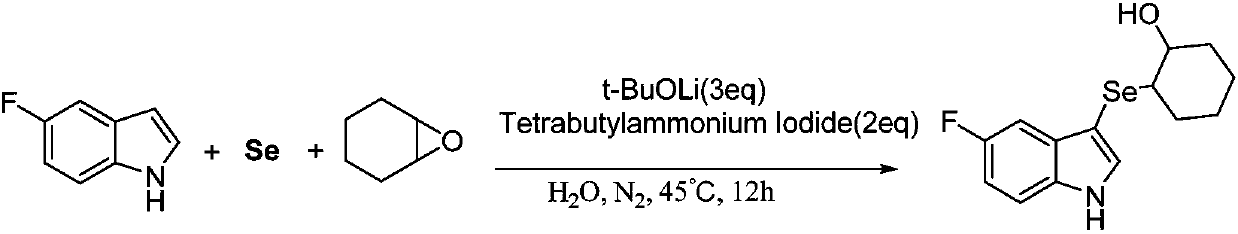
[0034] Synthesis of 2-(5-fluoro-3-indoleselenyl)-1-cyclohexyl alcohol:
[0035]
[0036] At room temperature, add a stir bar, selenium powder (0.8mmol), lithium tert-butoxide (1.2mmol), tetrabutylammonium iodide (0.8mmol), 5-fluoro-indole (0.4mmol) into a 25mL Schlenk tube, Then vacuumize, fill with nitrogen, back and forth vacuumize-fill nitrogen three times, add cyclohexene oxide (0.8mmol) and deionized water (2mL) under the protection of nitrogen, then stir at room temperature for 5min, and finally move to 45°C React in the heating tank, use TLC (or GC-MS) tracking detection during the reaction, after detection, the reaction is complete after 12 hours.
[0037] After the reaction is completed and the reaction mixture is cooled, the reaction mixture is first diluted with 15 mL of water, then extracted three times with 45 mL of ethyl acetate, and the organic phase is collected three times, then dried with anhydrous sodium sulfate, filtered, concentrated under reduced press...
Embodiment 3
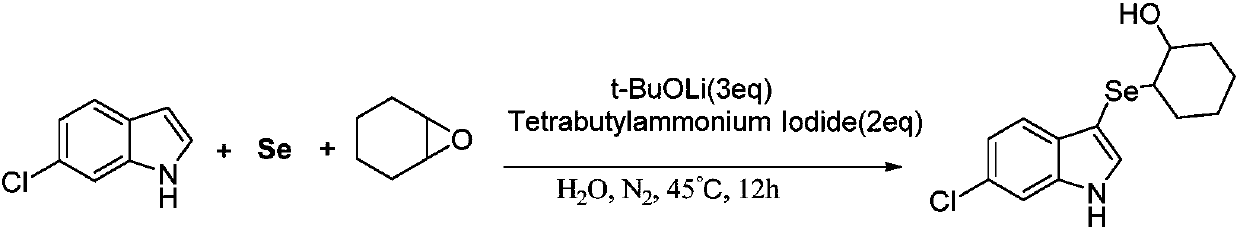
[0044] Synthesis of 2-(6-chloro-3-indoleselenyl)-1-cyclohexyl alcohol:
[0045]
[0046]At room temperature, add a stir bar, selenium powder (0.8mmol), lithium tert-butoxide (1.2mmol), tetrabutylammonium iodide (0.8mmol), 6-chloro-indole (0.4mmol) into a 25mL Schlenk tube, Then vacuumize, fill with nitrogen, back and forth vacuumize-fill nitrogen three times, add cyclohexene oxide (0.8mmol) and deionized water (2mL) under the protection of nitrogen, then stir at room temperature for 5min, and finally move to 45°C React in the heating tank, use TLC (or GC-MS) tracking detection during the reaction, after detection, the reaction is complete after 12 hours.
[0047] After the reaction is completed and the reaction mixture is cooled, the reaction mixture is first diluted with 15 mL of water, then extracted three times with 45 mL of ethyl acetate, and the organic phase is collected three times, then dried with anhydrous sodium sulfate, filtered, concentrated under reduced pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com