Synthesis method of thiosulfonate compound
A technology of thiosulfonic acid ester and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of large environmental damage and cannot be applied to industrial production, etc., and achieves the effects of low reaction temperature and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] This embodiment is a specific example of synthesizing thio-p-tolylbenzenesulfonate.
[0068] Weigh 0.6mmol benzenesulfonyl hydrazide, 0.4mmol p-methylthiophenol, 0.2mmol sodium iodide and 2.5mL acetonitrile into a 15mL pressure-resistant reaction tube, then add 1.0mmol peroxy tert-butanol ( TBHP), adding a magnetic stirrer, stirring and reacting at 25°C for 6h, please refer to formula 1 for the chemical equation of this embodiment.
[0069] After the reaction was completed, acetonitrile was distilled off under reduced pressure at -0.090MPa and 40°C, and then 50 mL of dichloromethane was used for suction filtration under reduced pressure, and the obtained filtrate was distilled under reduced pressure at -0.085MPa and 35°C to remove methylene chloride, Purify by silica gel column chromatography with 200-300 mesh silica gel powder, the eluent is petroleum ether:ethyl acetate=50:1, and the yield of the obtained product is 100%.
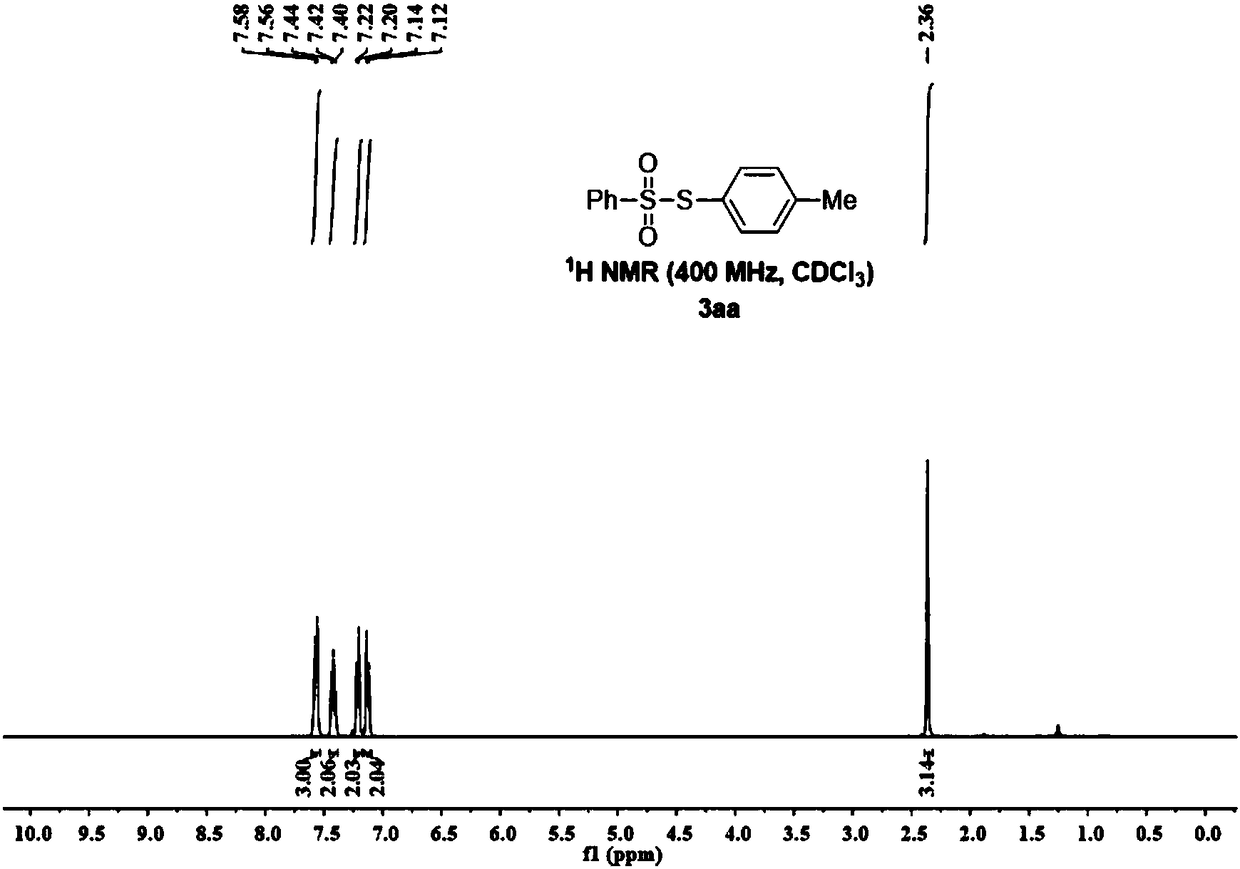
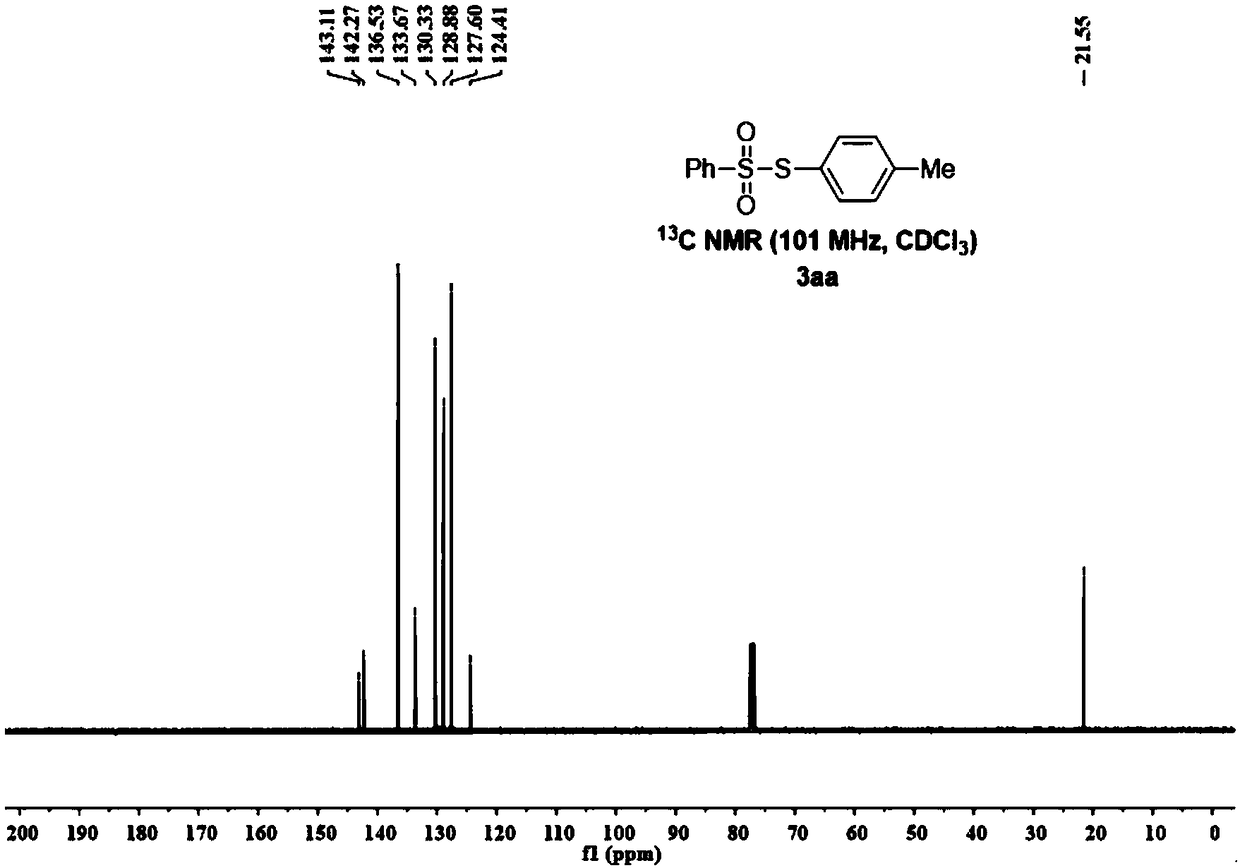
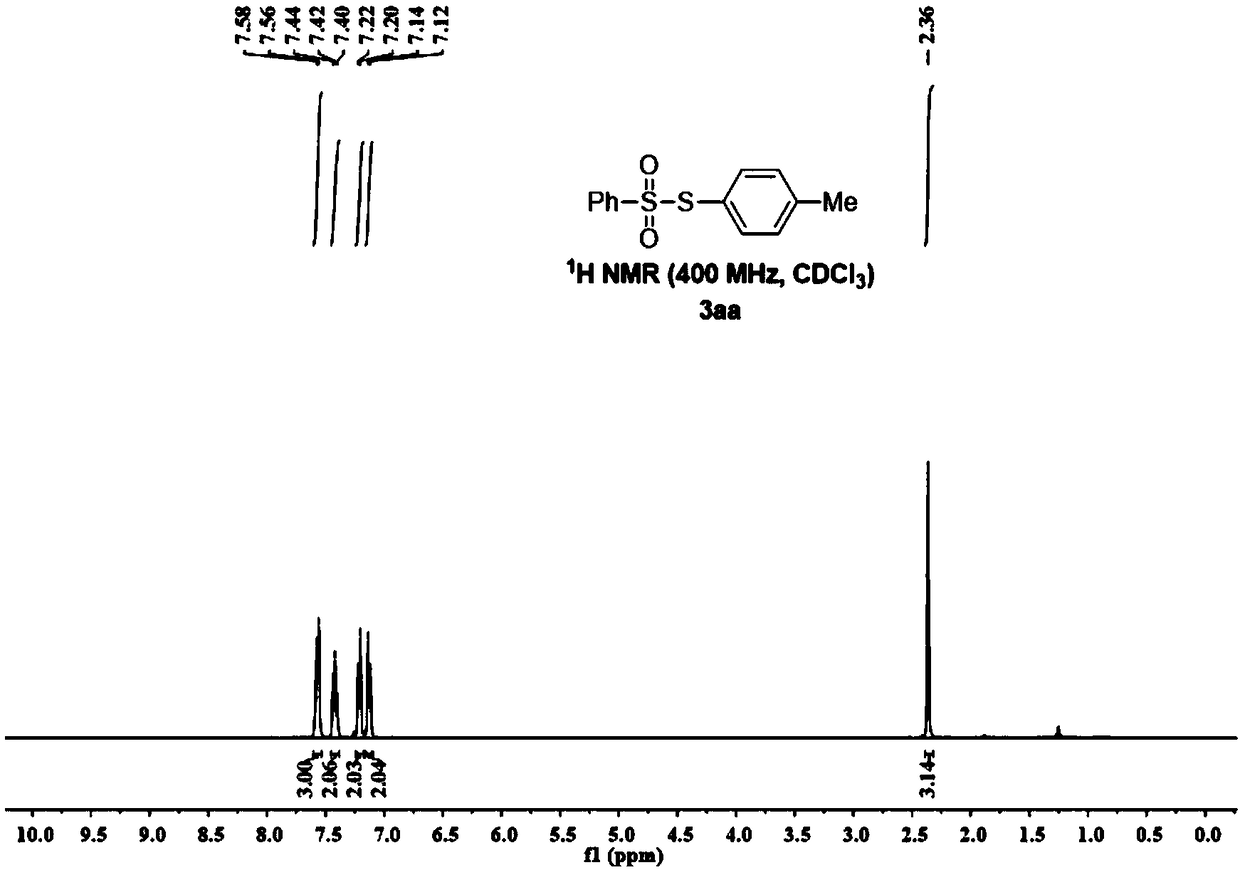
[0070] figure 1 , figure 2 It is the NMR...
Embodiment 2
[0074] This embodiment is a specific example of synthesizing thio-p-tolylbenzenesulfonate.
[0075] Weigh 0.6mmol benzenesulfonyl hydrazide, 0.4mmol p-methylthiophenol, 0.2mmol sodium iodide and 2.5mL nitromethane into a 15mL pressure-resistant reaction tube, then add 1.0mmol tert-butyl peroxide dropwise into the reaction tube Alcohol (TBHP), adding a magnetic stirrer, stirring and reacting at 25° C. for 8 hours, please refer to formula 2 for the chemical equation of this embodiment.
[0076] After the reaction is over, carry out vacuum distillation at -0.095MPa and 40°C to remove nitromethane, then use 50mL of dichloromethane to carry out vacuum filtration, and the obtained filtrate is to carry out vacuum distillation at -0.085MPa and 35°C to remove dichloromethane , and then purified by silica gel column chromatography with 200-300 mesh silica gel powder, the eluent is petroleum ether:ethyl acetate=50:1, and the yield of the product obtained is 100%.
[0077] image 3 , ...
Embodiment 3
[0081] This embodiment is the specific embodiment of synthetic thio-p-tolylbenzenesulfonate
[0082] Weigh 0.6mmol of benzenesulfonyl hydrazide, 0.4mmol of p-methylthiophenol, 0.2mmol of sodium iodide and 2.5mL of 1,4dioxane (1,4dioxane) into a 15mL pressure-resistant reaction tube, and then pour into the reaction tube Add 1.0 mmol of tert-butanol peroxy (TBHP) dropwise, add a magnetic stirrer, and stir the reaction at 25° C. for 10 h. Please refer to Formula 3 for the chemical formula of this example.
[0083] After the reaction is over, carry out vacuum distillation at -0.095MPa and 45°C to remove 1,4-dioxane, then carry out vacuum filtration with 50mL of dichloromethane, and depressurize the obtained filtrate at -0.085MPa and 35°C The dichloromethane was distilled off, and purified by silica gel column chromatography with 200-300 mesh silica gel powder, the eluent was petroleum ether: ethyl acetate = 50:1, and the yield of the obtained product was 96%.
[0084] Figure 5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com