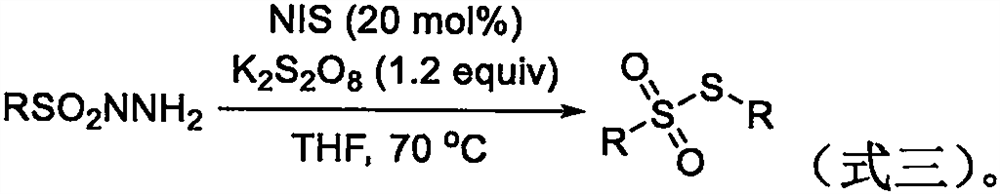Patents
Literature
40 results about "Thiosulfonic Acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
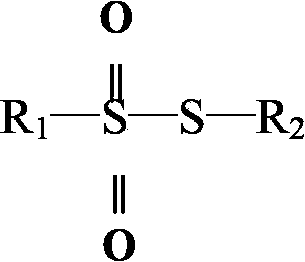
Inorganic or organic oxy acids of sulfur which contain the general formula RS2O2H.
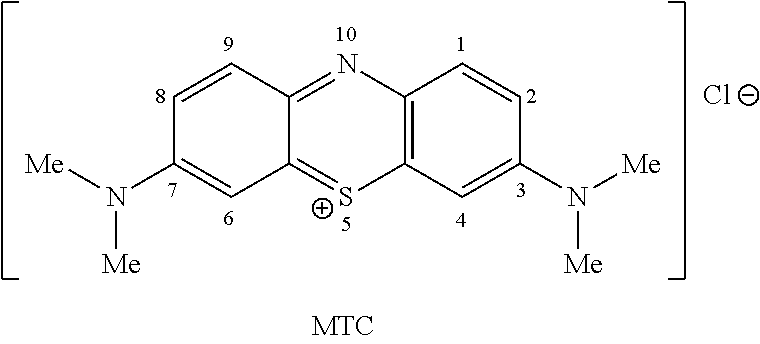
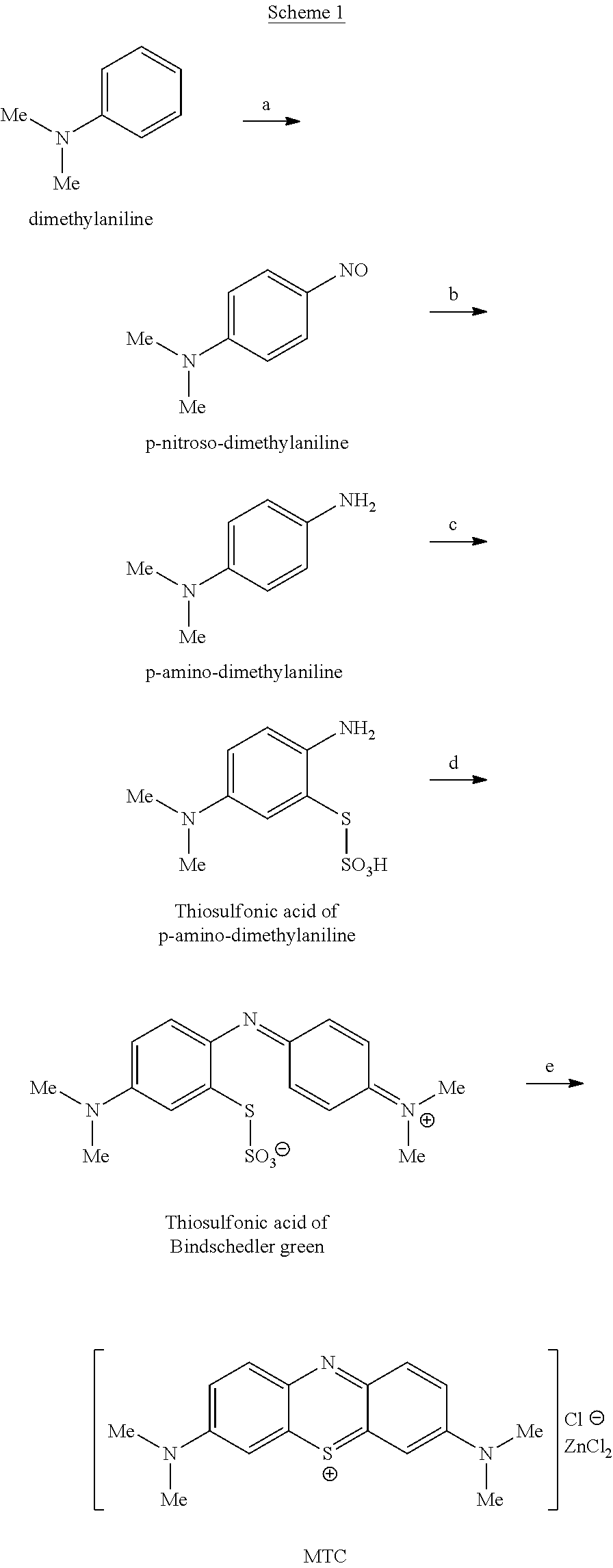
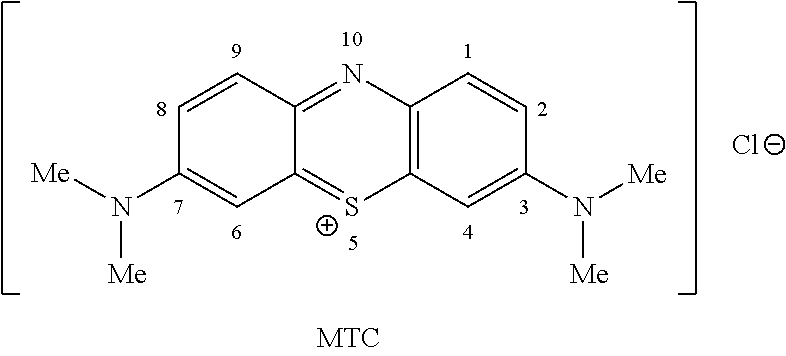
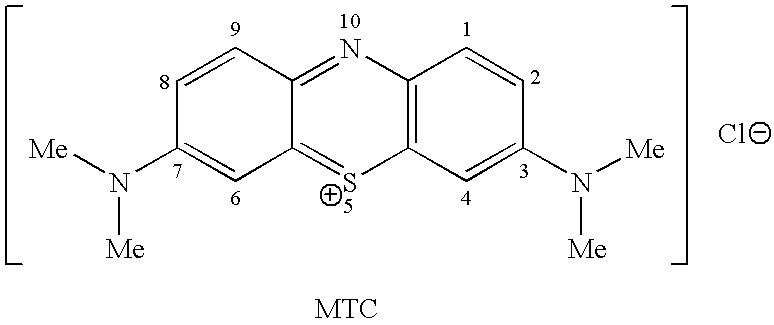
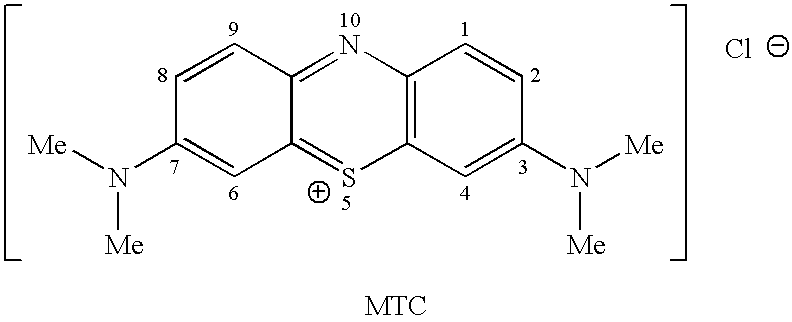
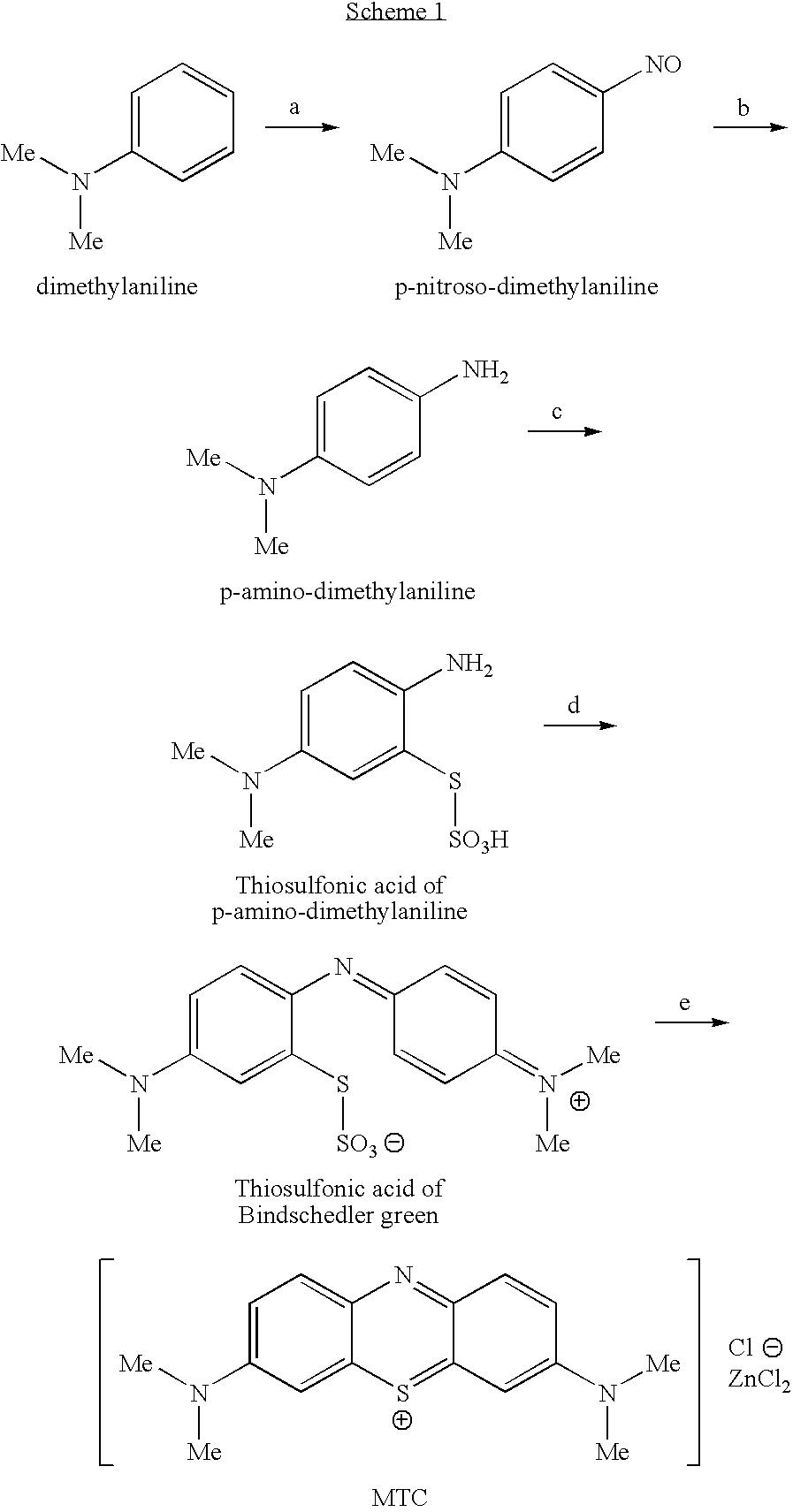
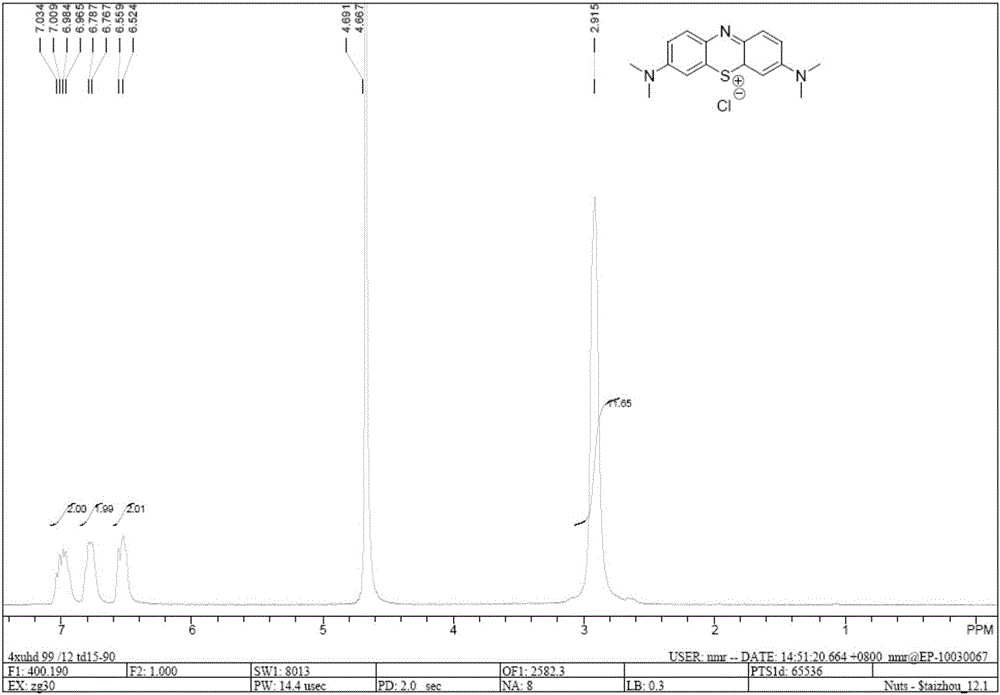
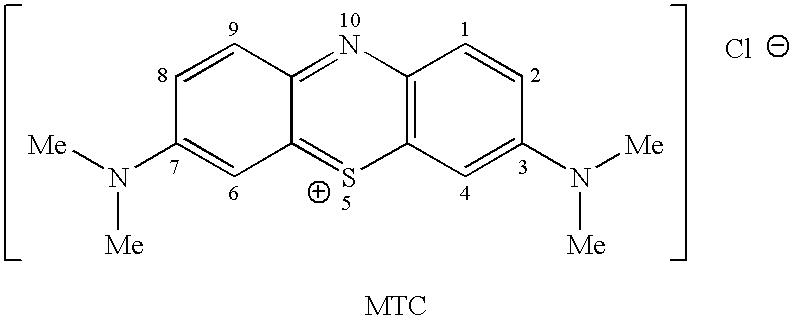
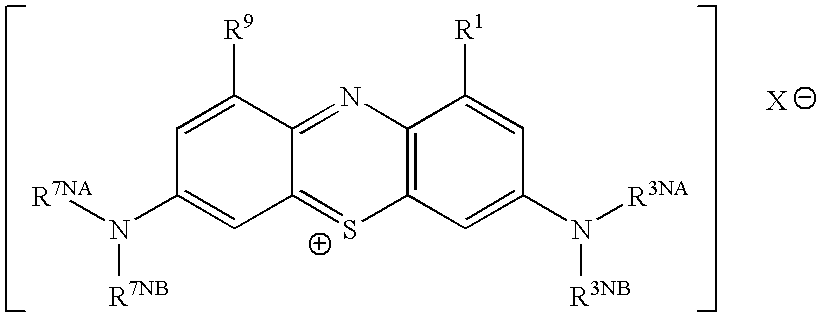
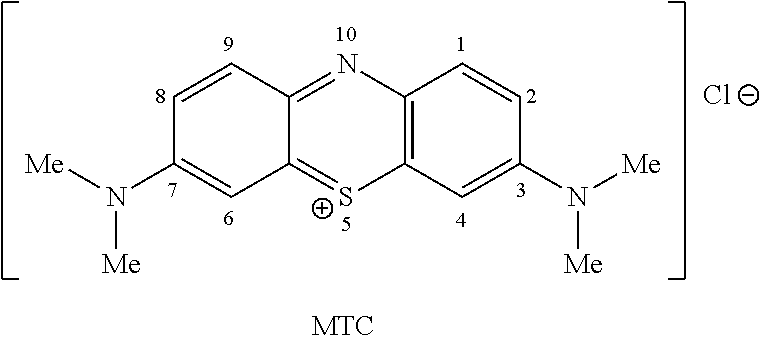
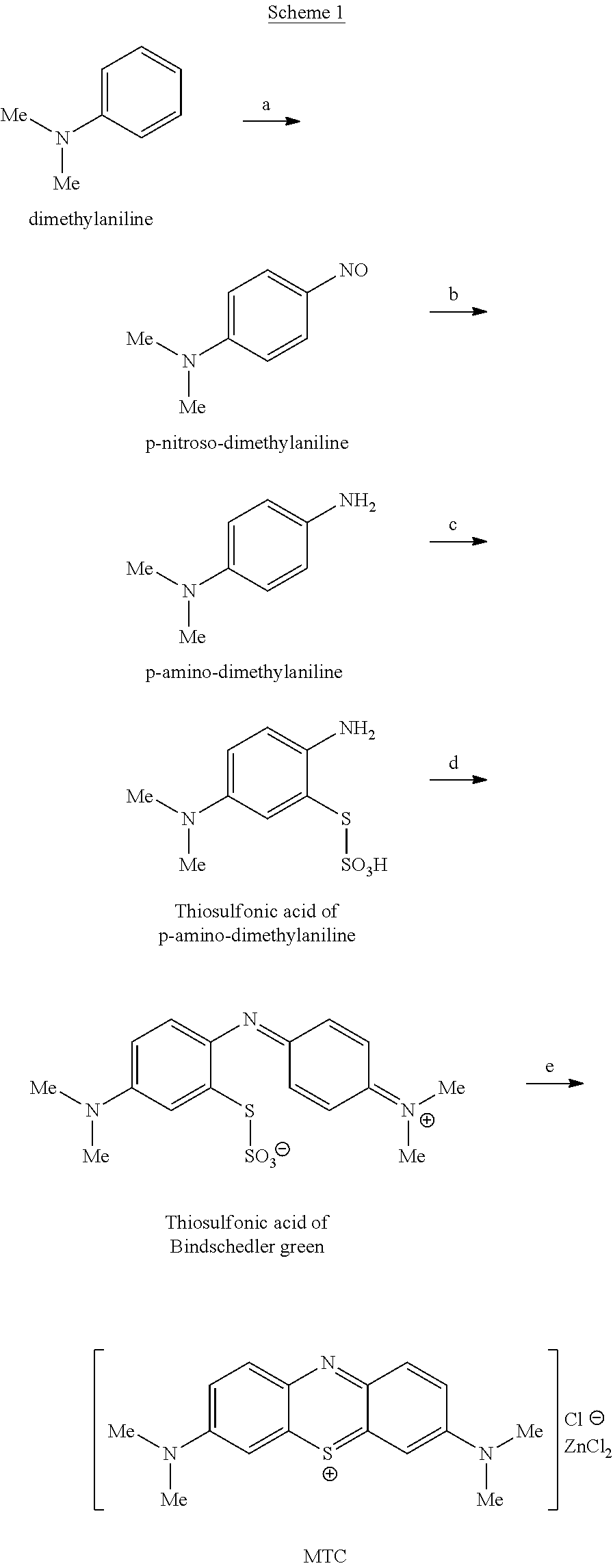
Methods of chemical synthesis and purification of diaminophenothiazinium compounds including methylthioninium chloride (MTC)
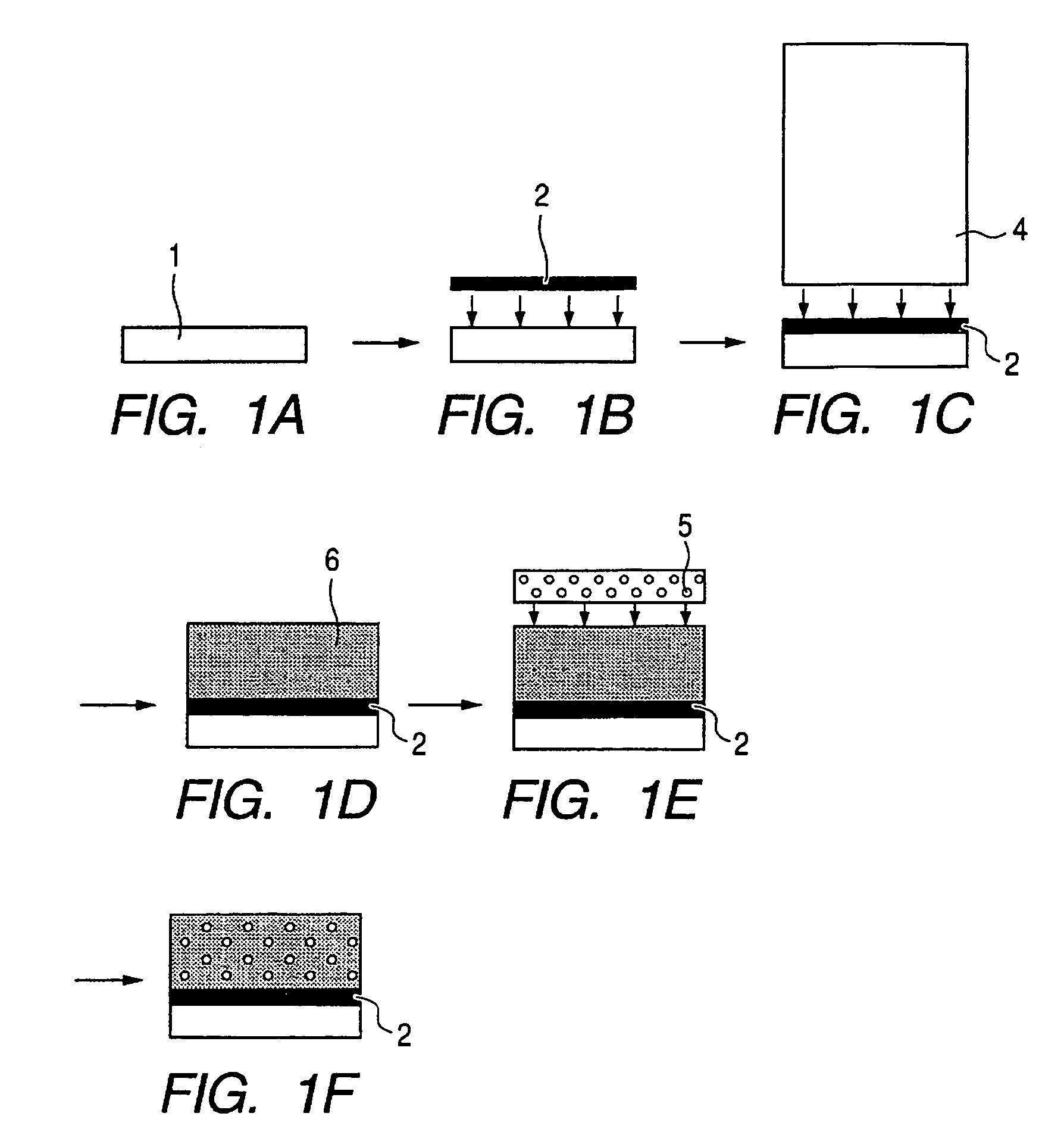
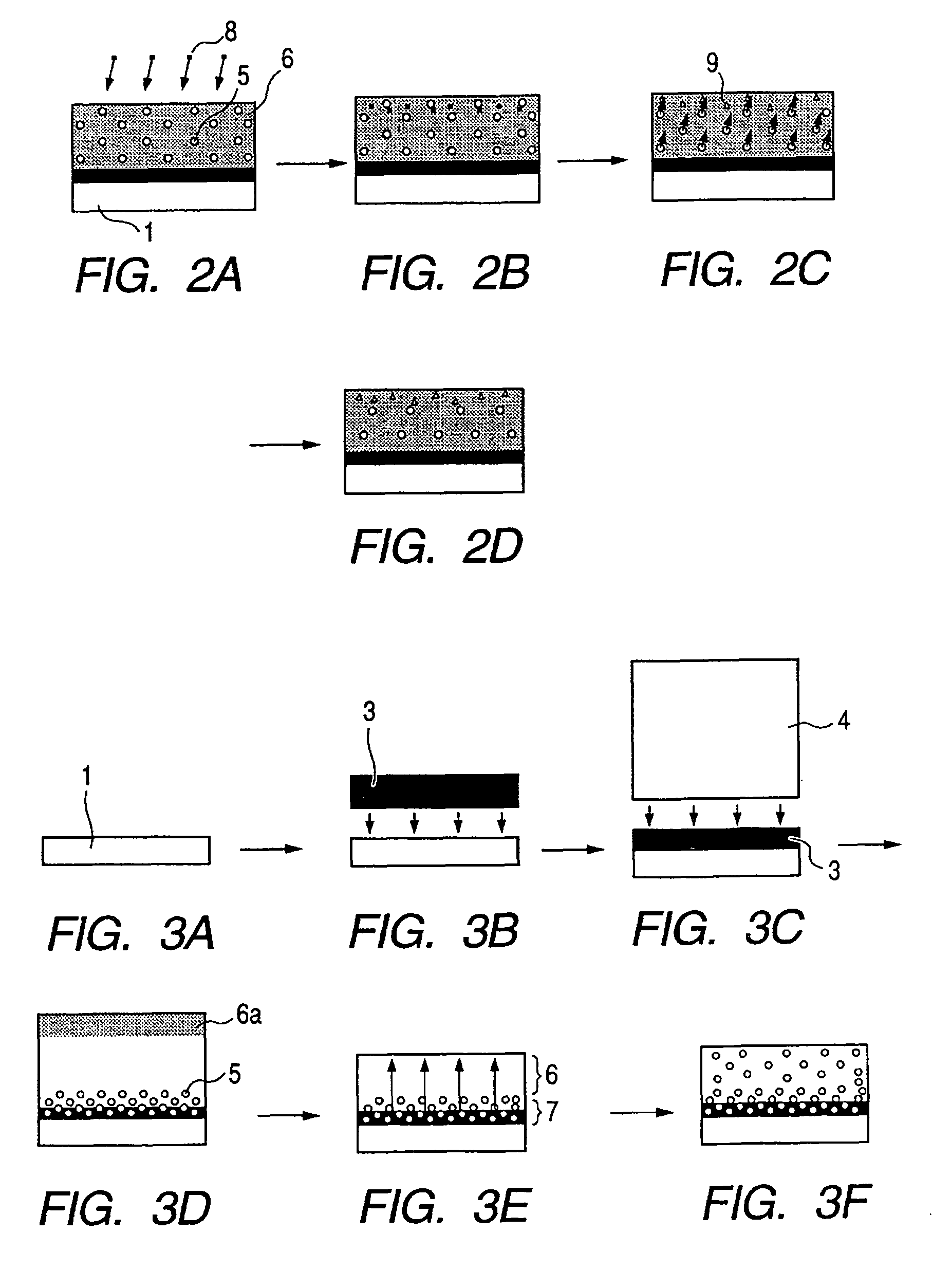
This invention pertains generally to the field of chemical synthesis and purification, and more specifically to methods of synthesizing and purifying certain 3,7-diaminophenothiazin-5-ium compounds (referred to herein as “diaminophenothiazinium compounds”) including Methythioninium Chloride (MTC) (also known as Methylene Blue). In one embodiment, the method comprises the steps of, in order: nitrosylation (NOS); nitrosyl reduction (NR); thiosulfonic acid formation (TSAF); oxidative coupling (OC); Cr(VI) reduction (CR); isolation and purification of zwitterionic intermediate (IAPOZI); ring closure (RC); chloride salt formation (CSF); one of: sulphide treatment (ST); dimethyldithiocarbamate treatment (DT); carbonate treatment (CT); ethylenediaminetetraacetic acid treatment (EDTAT); organic extraction (OE); and recrystallisation (RX). The present invention also pertains to the resulting (high purity) compounds, compositions comprising them (e.g., tablets, capsules), and their use in methods of inactivating pathogens, and methods of medical treatment and diagnosis, etc., for example, for tauopathies, Alzheimer's disease (AD), skin cancer, melanoma, viral diseases, bacterial diseases, or protozoal diseases.
Owner:WISTA LAB LTD
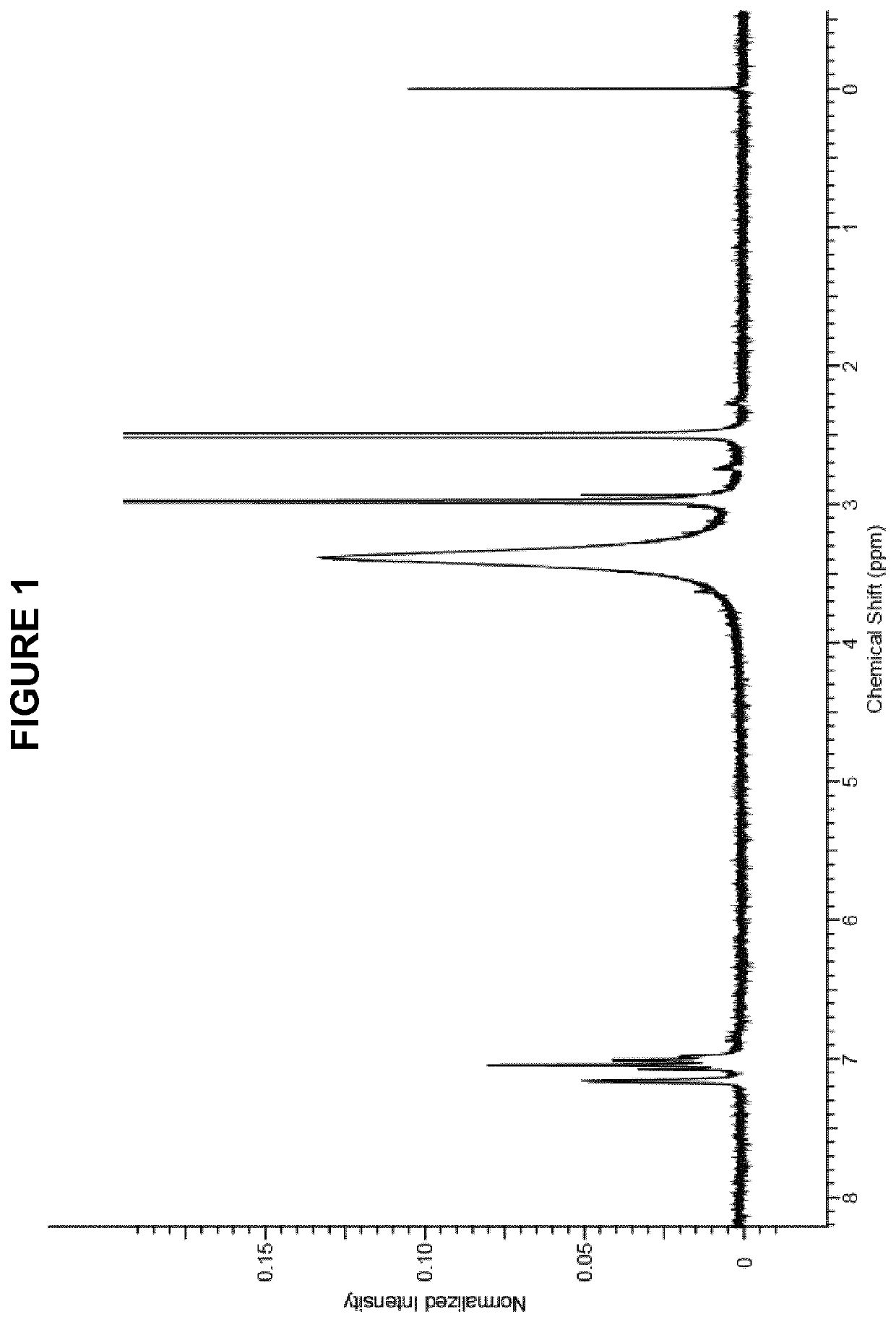
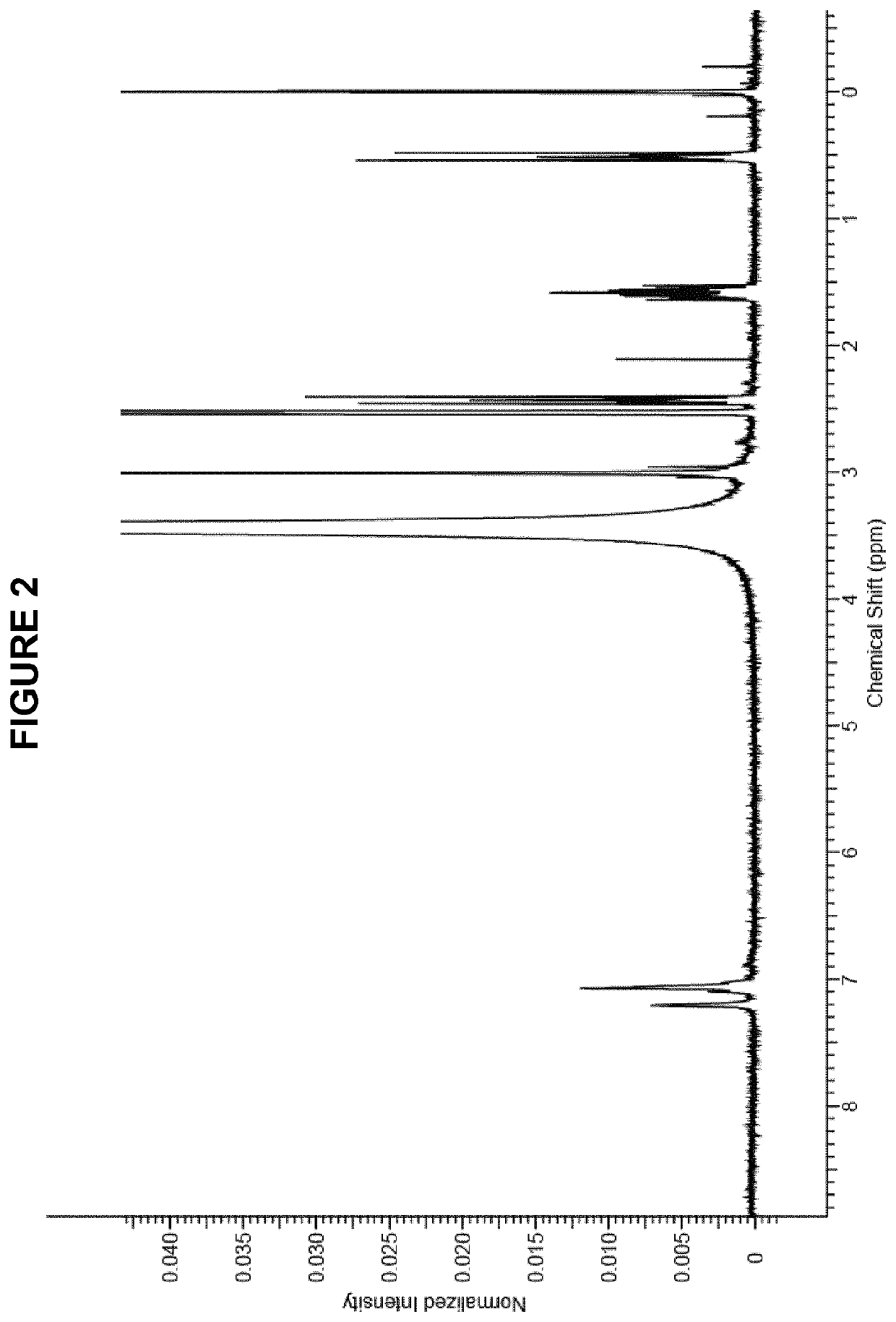
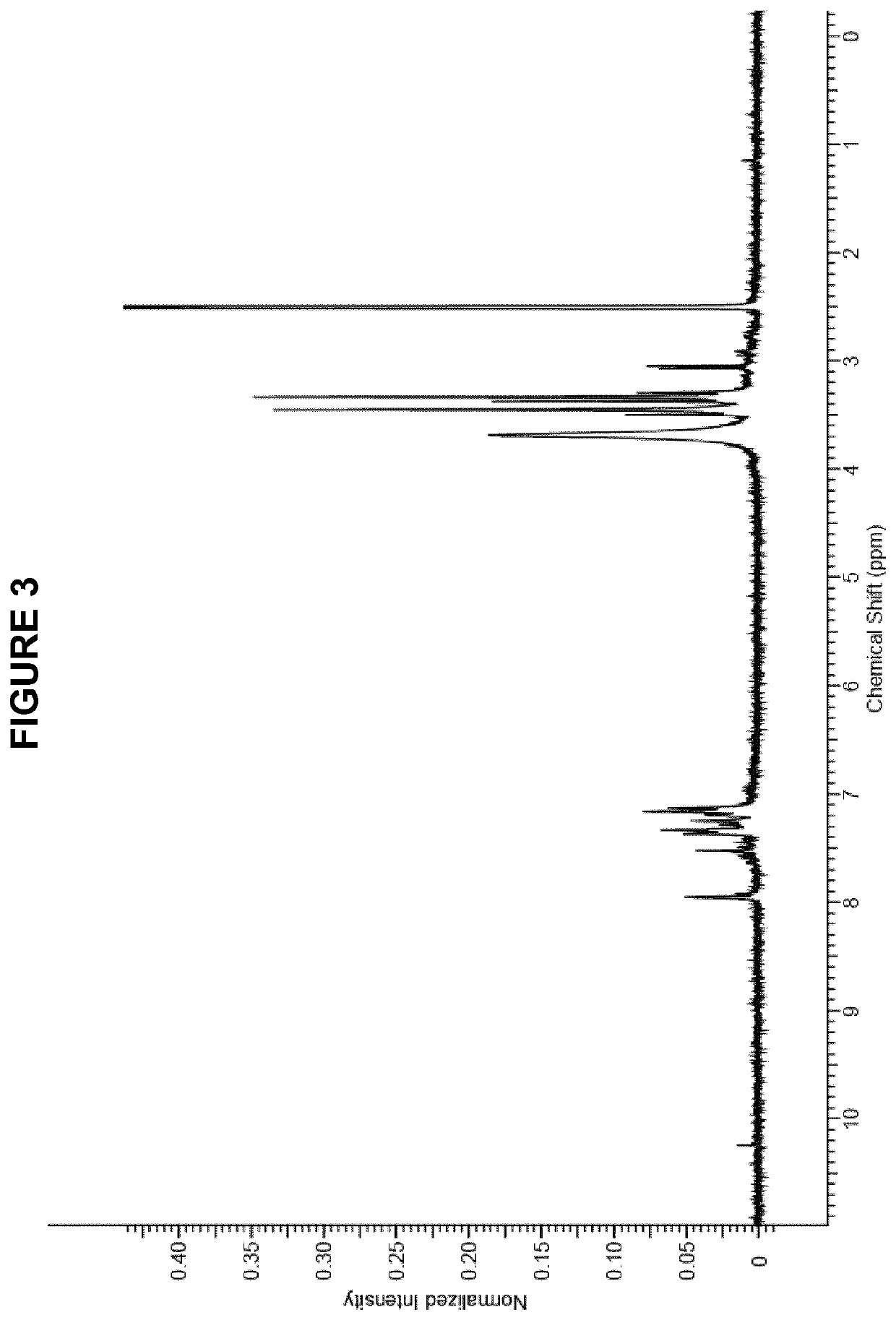
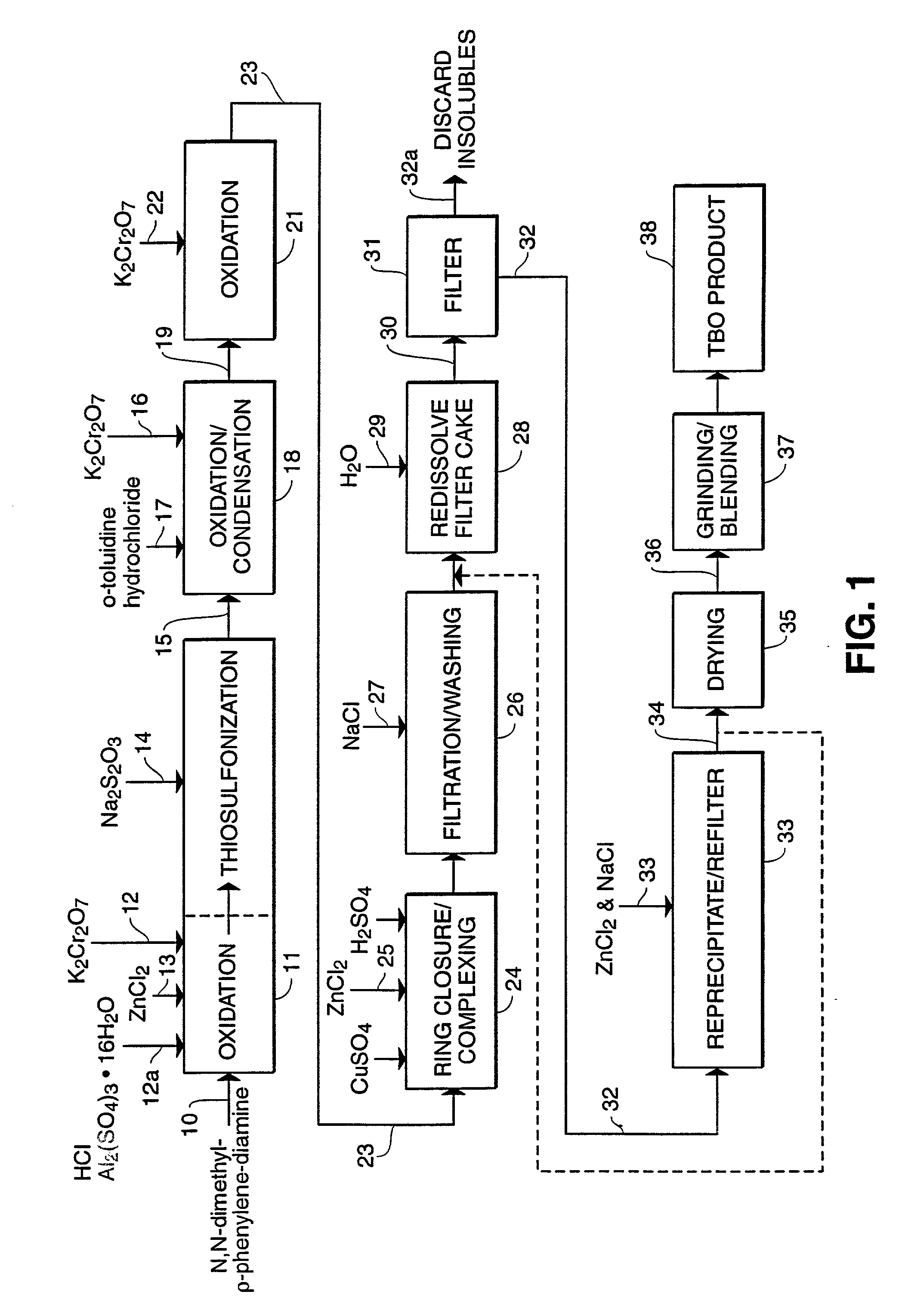
Process for manufacture of in vivo stain composition
InactiveUS6194573B1Organic chemistryLuminescence/biological staining preparationThiosulfonic AcidsIn vivo
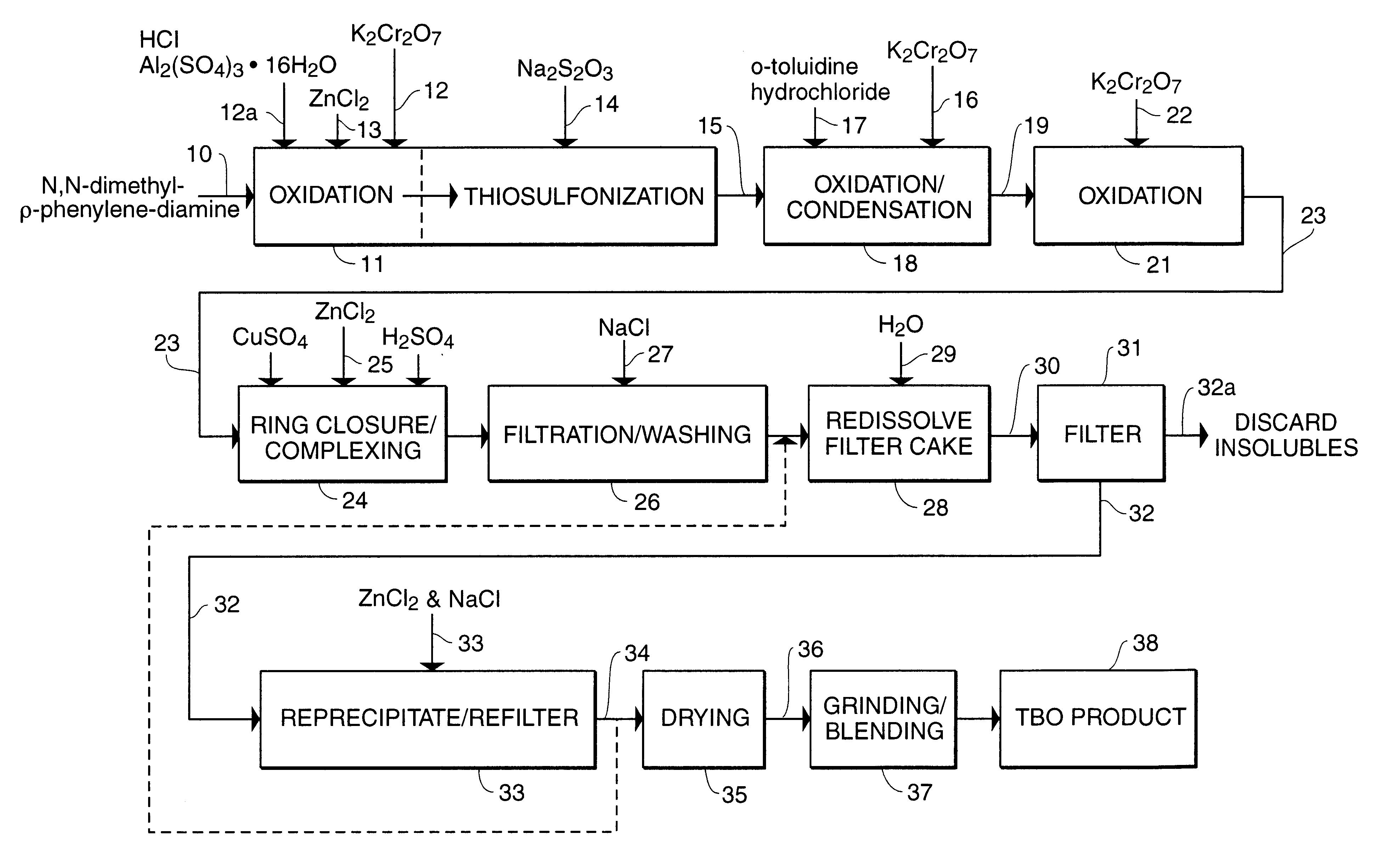
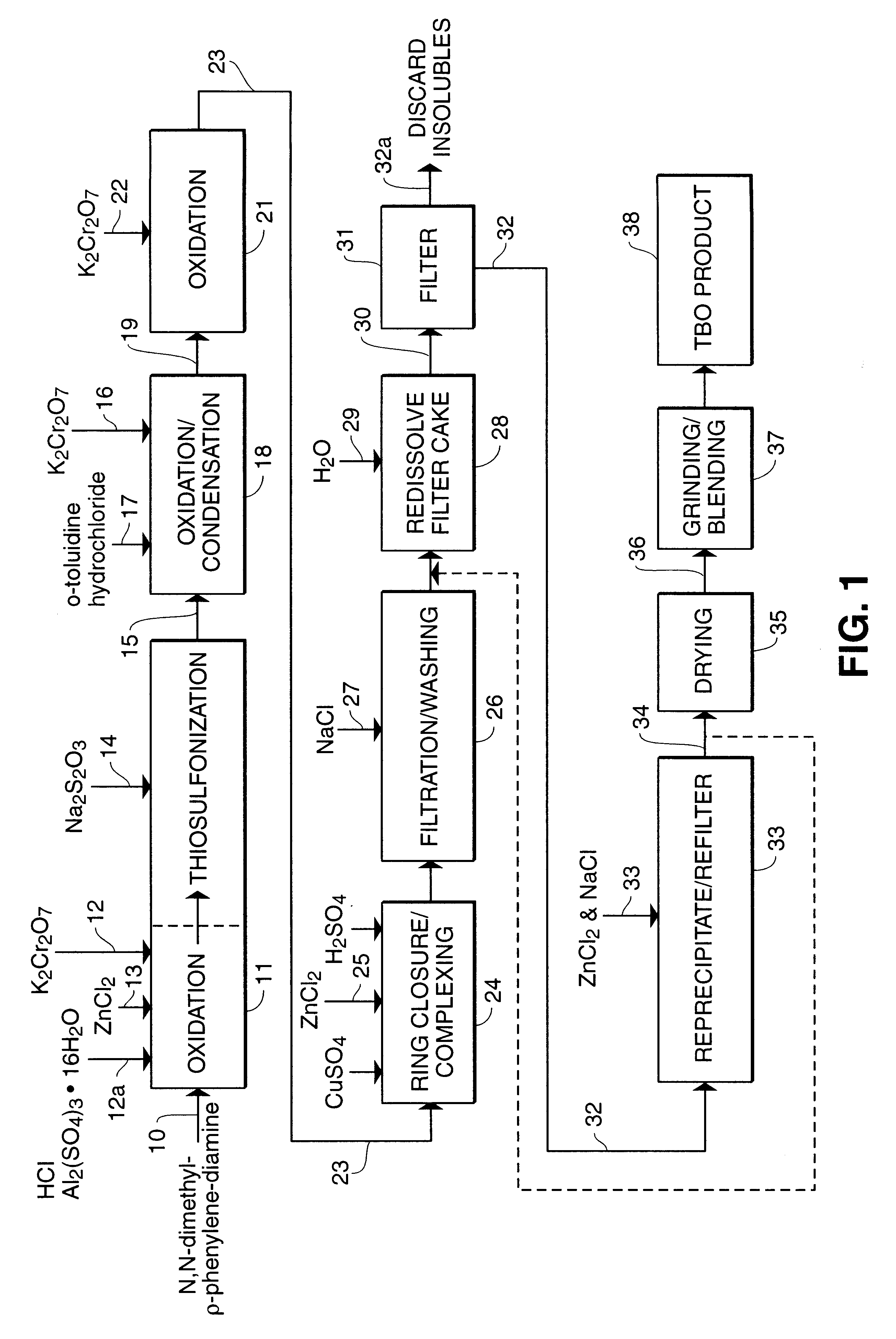
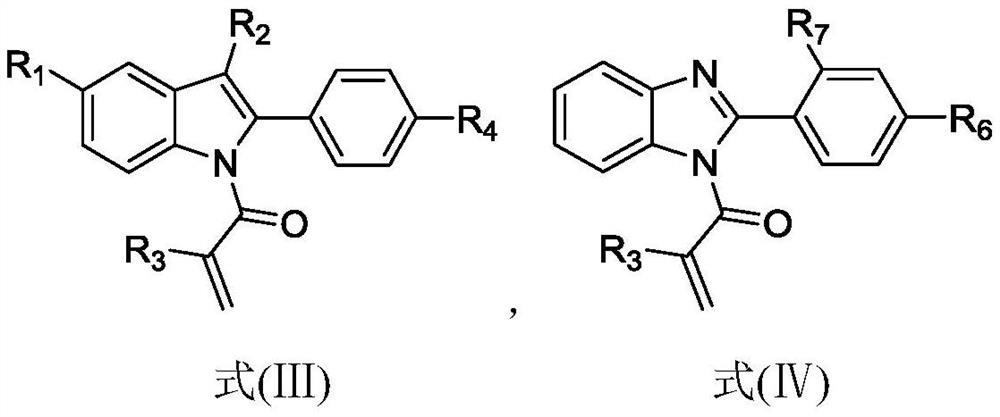
A process for synthesizing 2-amino-5-dimethlyaminophenyl thiosulfonic acid comprises the step of oxidizing N,N'-dimethyl-rho-phenylene-diamine in the presence of a source of thiosulfate ions, while maintaining the temperature of the reaction mixture not higher than about 10° C. This compound is useful as an intermediate in the synthesis of toluidine blue O.A process for manufacturing toluidine blue O with improved yield, includes the step of preparing the intermediate 2-amino-5-diethylaminopropyl thiosulfonic acid according to the above described procedure.
Owner:DEN MAT HLDG
Article having microporous body part, production method of ink medium, diffusion method of sulfur-containing organic acid into microporous layer, production method of article having meicroporous body part, and inkjet recording medium produced therefrom
InactiveUS7867586B2Eliminate yellowingGood prevention effectCoatingsThermographyDiffusion methodsOrganic acid
Provided is an inkjet recording medium in which hydrated alumina and a sulfinic acid compound or thiosulfonic acid compound coexist in a pigment in an ink receiving layer, and which can prevent white-background yellowing during storage in a resin file holder or the like and ensure printing quality at the same time. The ink receiving layer of the inkjet recording medium contains the sulfinic acid compound or thiosulfonic acid compound, which functions to prevent yellowing, in a salt form or in a free form so as to be diffusible.
Owner:CANON KK
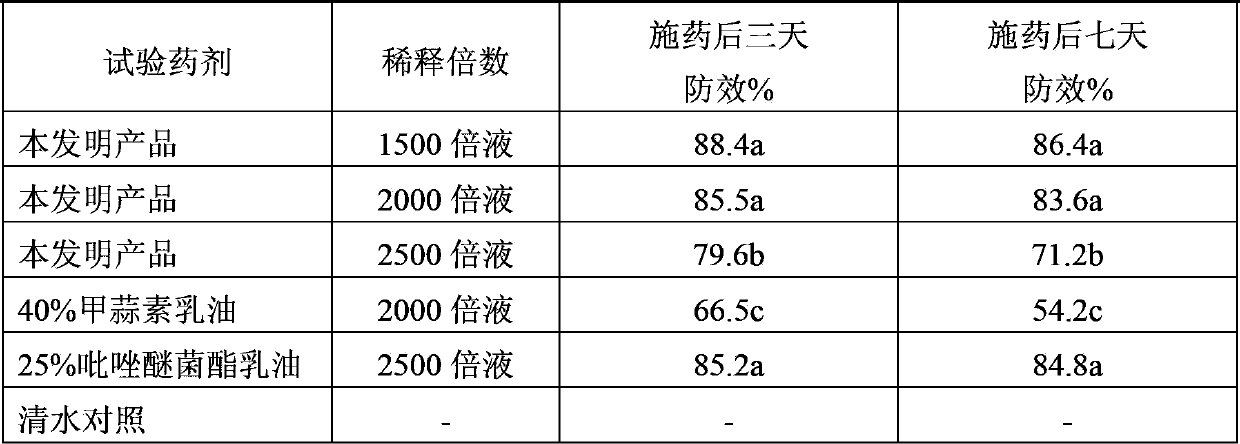
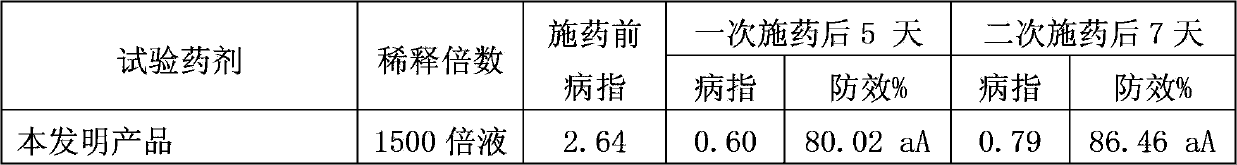
Pesticide composition containing thiosulfonic acid ester fungicide
The invention discloses a pesticide composition containing a thiosulfonic acid ester fungicide. The pesticide composition contains 10-60% of thiosulfonic acid ester, powders of 1-40 parts of methoxy-crylic acid fungicide, a soluble concentrate, a microemulsion, an emulsion in water, a suspending agent or missible oil. The pesticide composition is a compound composition of the thiosulfonic acid ester fungicide and the methoxy-crylic acid fungicide, high synergetic effect can be generated, and the capabilities of sterilization, disease prevention and healing can be reinforced, so that the crop can be prevented from falling ill in the growth process, and the yield and the quality are improved; the pesticide composition has a broad-spectrum sterilization capability, can prevent the diseases caused by various germs, and particularly plays the role of forcefully killing the moulds; and according to the pesticide composition, the use dose is reduced relatively, and the using cost is lowered relatively; the pesticide composition is processed to be a water-based environment-friendly agent, such as the emulsion in water, the microemulsion, a suspending agent and a suspension emulsion, the use dosage of the organic solvent is reduced, and the safety of the pesticide composition is improved.
Owner:夏建中
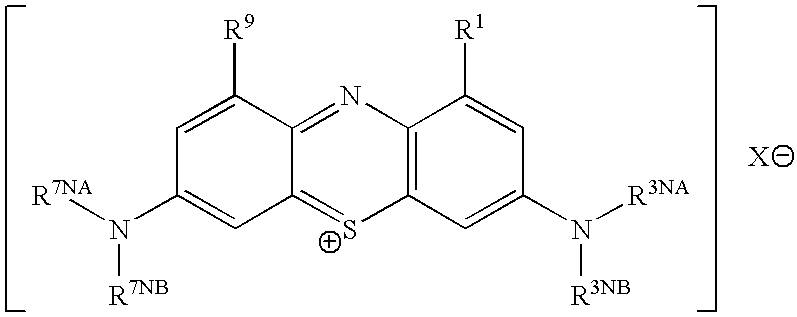
Methods of chemical synthesis and purification of diaminophenothiazinium compounds including methylthioninium chloride (MTC)
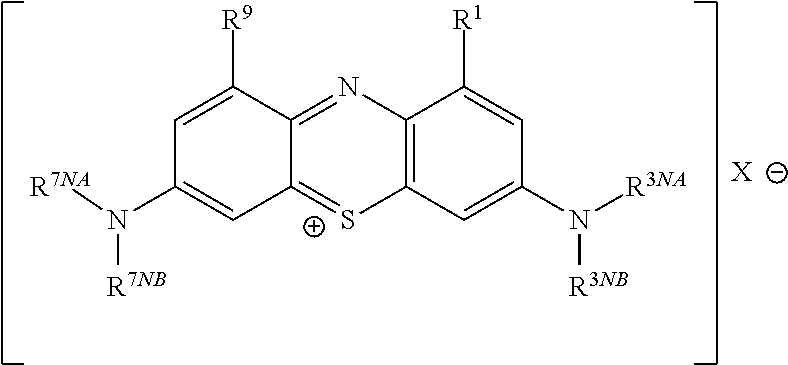
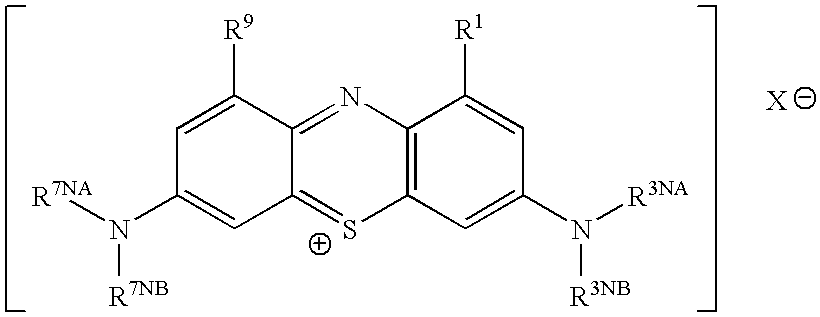
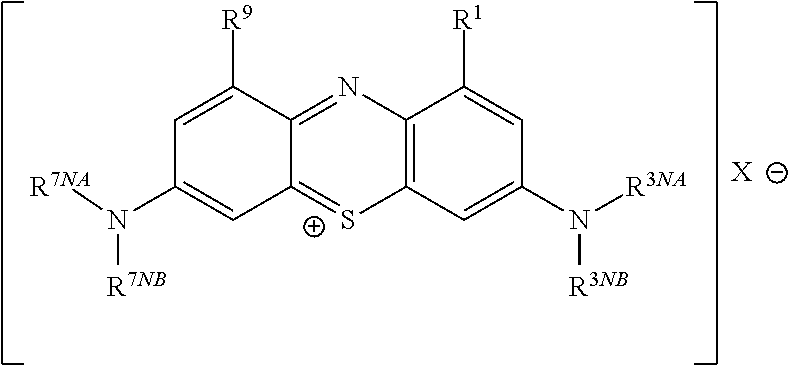
This invention pertains generally to the field of chemical synthesis and purification, and more specifically to methods of synthesizing and purifying certain 3,7 diamino-phenothiazin-5-ium compounds (referred to herein as “diaminophenothiazinium compounds”) including Methylthioninium Chloride (MTC) (also known as Methylene Blue). In one embodiment, the method comprises the steps of, in order: nitrosylation (NOS); nitrosyl reduction (NR); thiosulfonic acid formation (TSAF); oxidative coupling (OC); Cr(VI) reduction (CR); isolation and purification of zwitterionic intermediate (IAPOZI); ring closure (RC); chloride salt formation (CSF); one of: sulphide treatment (ST); dimethyldithiocarbamate treatment (DT); carbonate treatment (CT); ethylenediaminetetraacetic acid treatment (EDTAT); organic extraction (OE); and recrystallisation (RX). The present invention also pertains to the resulting (high purity) compounds, compositions comprising them (e.g., tablets, capsules), and their use in methods of inactivating pathogens, and methods of medical treatment and diagnosis, etc., for example, for tauopathies, Alzheimer's disease (AD), skin cancer, melanoma, viral diseases, bacterial diseases, or protozoal diseases. Wherein: each of R1 and R9 is independently selected from: —H; C1-4 alkenyl; and halogenated C1-4alkyl; each of R3NA and R3NB is independently selected from: C1-4 alkyl; C2-4alkenyl; and halogenated C4-1 alkyl; each of R7NA and R7NB is independently selected from: C1-4 alkyl; C2-4alkenyl; and halogenated C1-4 alkyl; and X is one or more anionic counter ions to achieve electrical neutrality.
Owner:WISTA LAB LTD
Article having microporous body part, production method of ink medium, diffusion method of sulfur-containing organic acid into microporous layer, production method of article having meicroporous body part, and inkjet recording medium produced therefrom
InactiveUS20060182906A1Eliminate riskEliminate effectiveCoatingsThermographyDiffusion methodsOrganic acid
Provided is an inkjet recording medium in which hydrated alumina and a sulfinic acid compound or thiosulfonic acid compound coexist in a pigment in an ink receiving layer, and which can prevent white-background yellowing during storage in a resin file holder or the like and ensure printing quality at the same time. The ink receiving layer of the inkjet recording medium contains the sulfinic acid compound or thiosulfonic acid compound, which functions to prevent yellowing, in a salt form or in a free form so as to be diffusible.
Owner:CANON KK
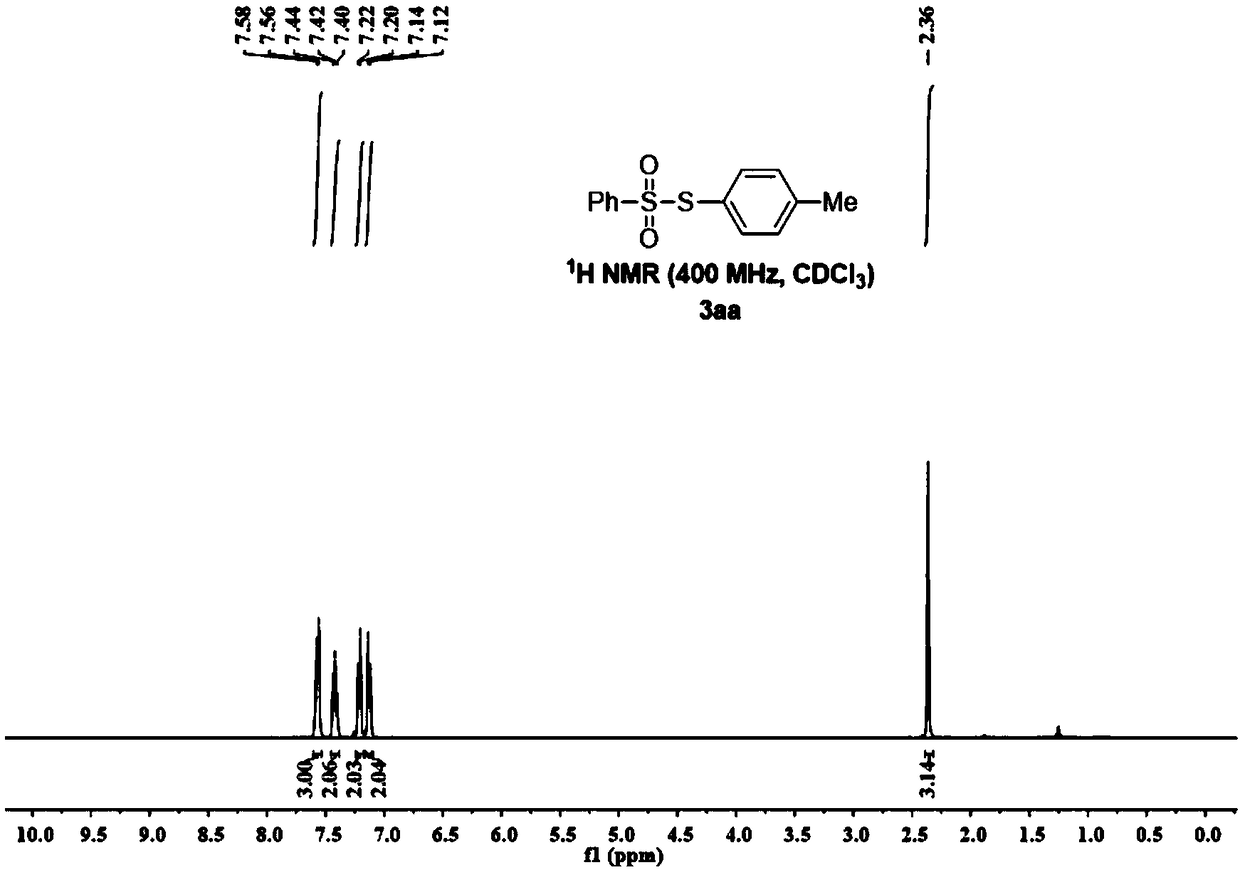
Synthesis method of thiosulfonate compound
ActiveCN108191729AImprove conversion rateNo pollutionOrganic chemistryOrganic synthesisThiosulfonic Acids
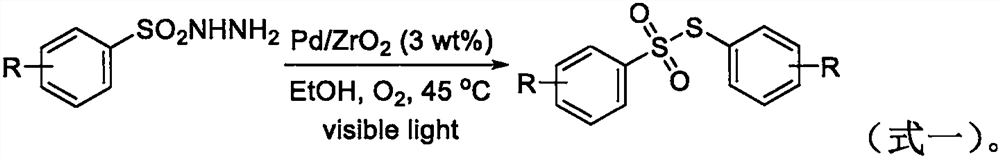
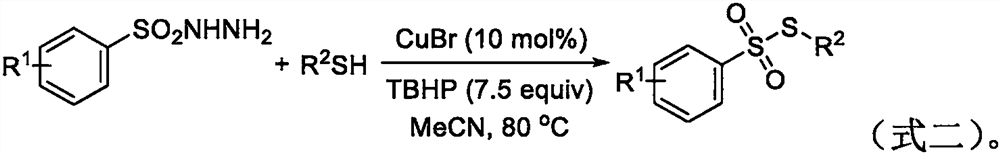
The invention belongs to the field of organic synthesis, and particularly relates to a synthesis method of a thiosulfonate compound. The invention provides the synthesis method of the thiosulfonate compound. The method comprises the following steps that methanesulfonohydrazide compounds, thiophenol compounds, oxidizing agents and catalysts are dissolved into a solvent for reaction; purification isperformed to obtain the thiosulfonate compound; oxidizing agents are any one kind or several kinds of materials from tertiary butanol peroxide and catalysts are sodium iodide, potassium iodide, ammonium iodide, iodine element and tetrabutylammonium iodide. Through experiment testing, the thiosulfonate compound prepared by the technical scheme provided by the invention has the advantages that no side reaction occurs; the yield can reach 84 to 100 percent; the conversion rate is high; meanwhile, the used catalysts and catalysts are environment-friendly compounds; the environment pollution cannot be caused; the reaction temperature is low; the method is suitable for industrial mass popularization. The defects that in the prior art, the damage to the environment in the asymmetric thiosulfonates synthesis process is great, and the application to industrial production cannot be realized are overcome.
Owner:GUANGDONG UNIV OF TECH
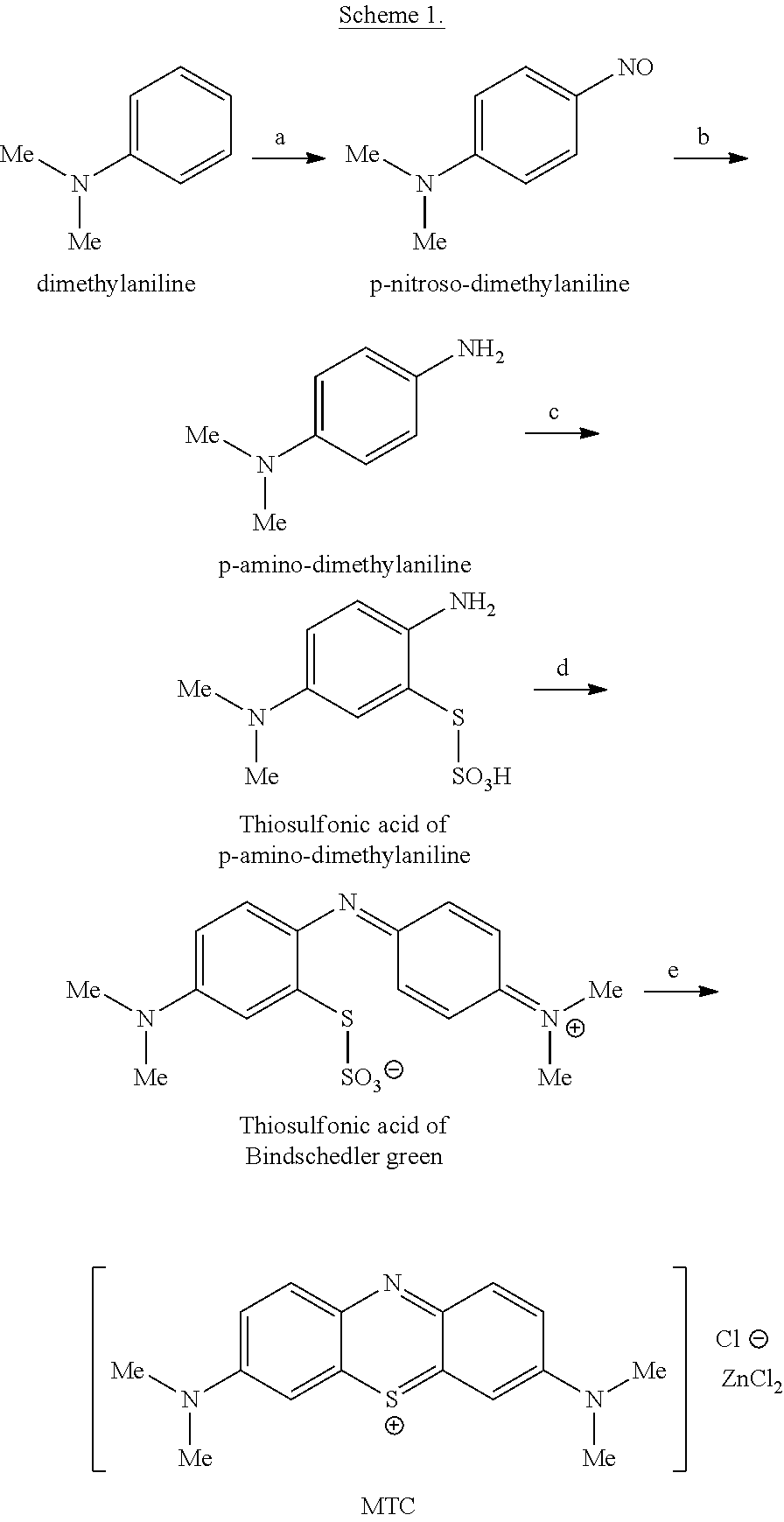
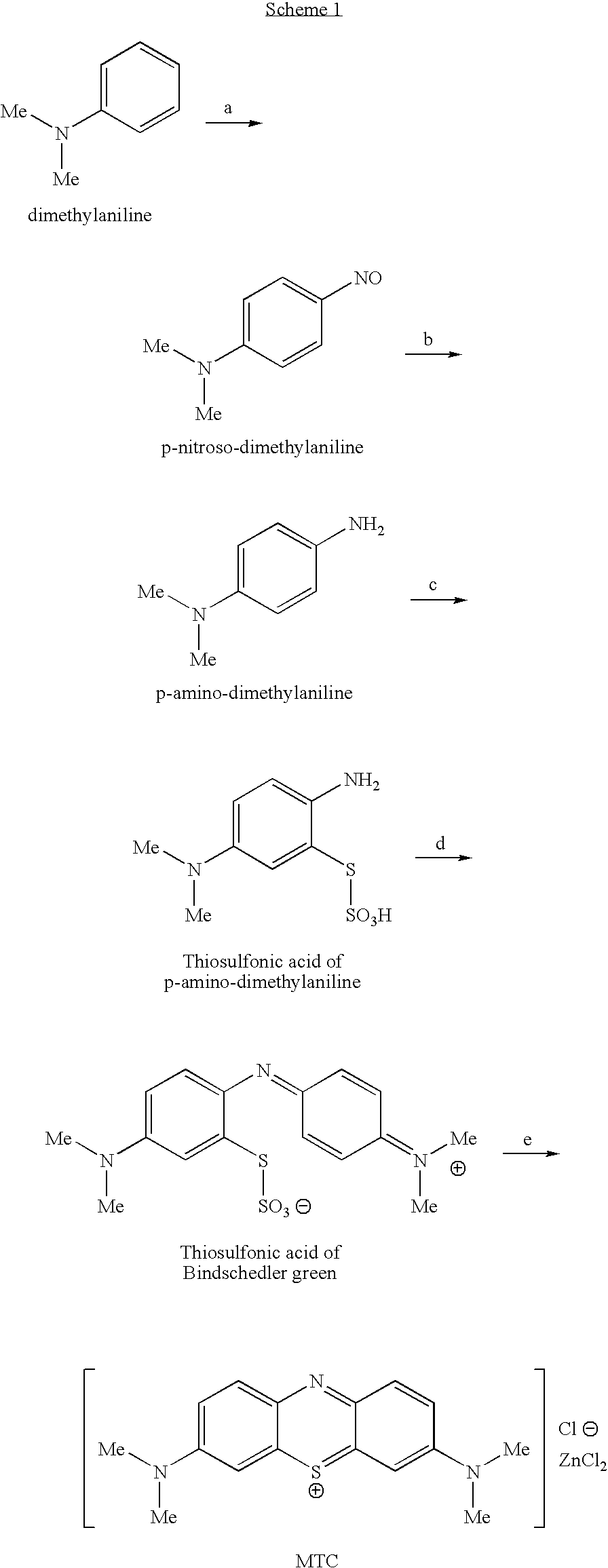
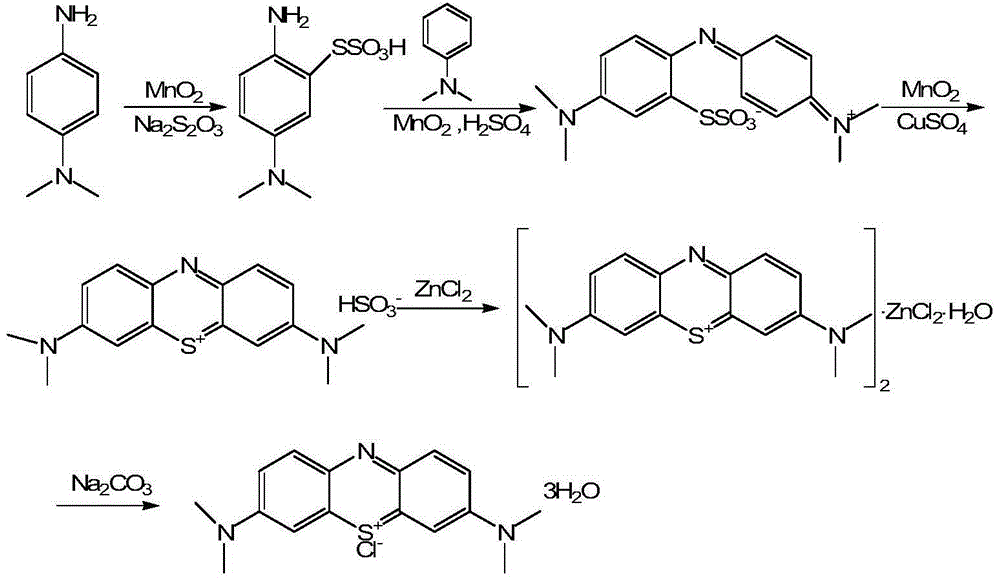
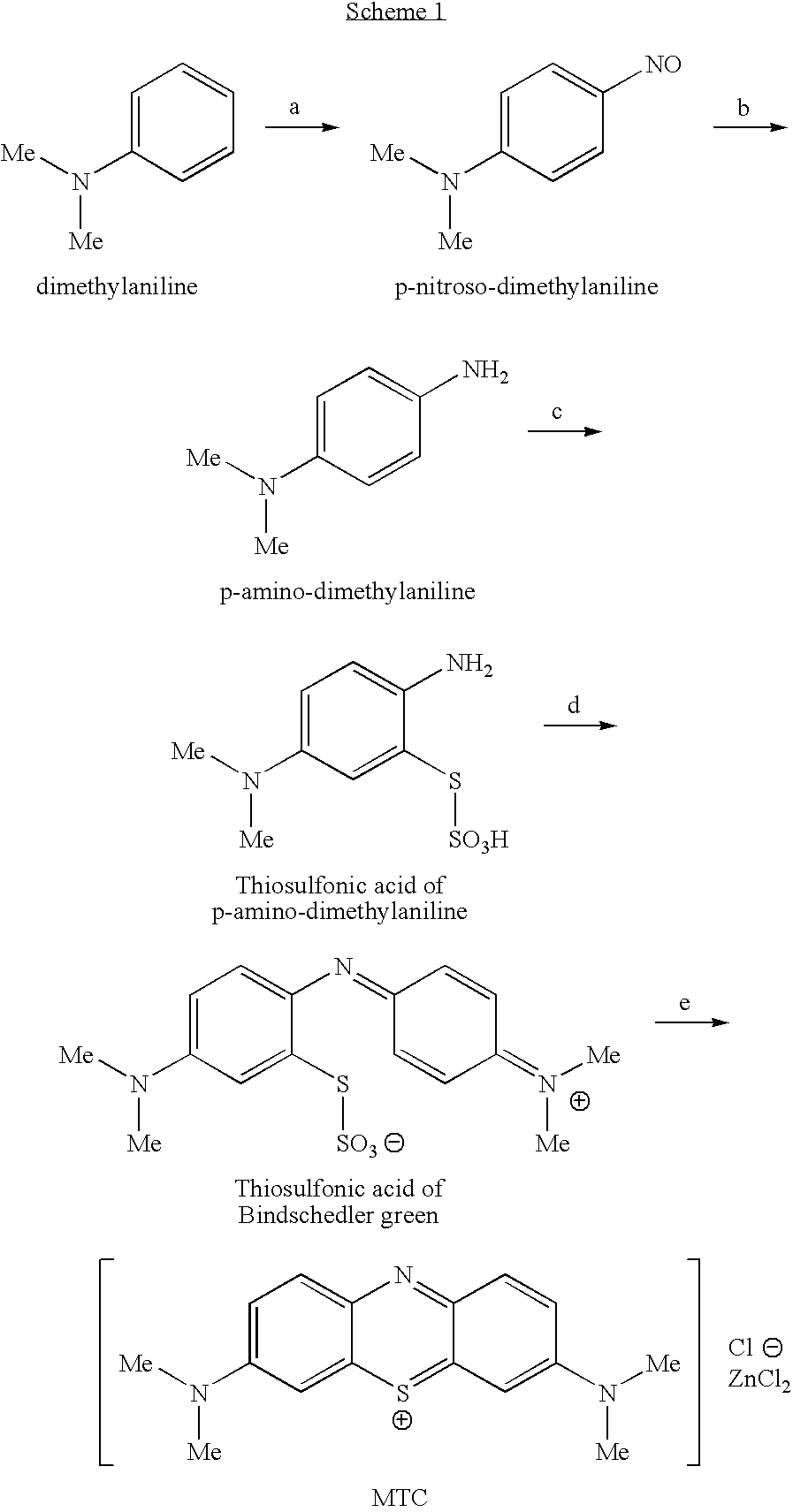
Preparation method of methylene blue
The invention discloses a preparation method of methylene blue, and belongs to the technical field of intermediate synthesis in fine chemical engineering. The preparation method comprises the following steps: in a condensed hydrochloric acid solution, carrying out nitrosation reactions between sodium nitrite and N,N-dimethylaniline so as to obtain an intermediate namely p-nitroso-N,N-dimethylaniline; subjecting p-nitroso-N,N-dimethylaniline to hydrogenation reduction to prepare p-amino-N,N-dimethylaniline; oxidizing p-amino-N,N-dimethylaniline, then adding sodium thiosulfate to carry out addition reactions to prepare 2-amino-5-dimethylaminophenyl thiosulfonic acid, adding N,N-dimethylaniline into 2-amino-5-dimethylaminophenyl thiosulfonic acid to carry out oxidative condensation reactions to generate bis(4-dimethylaminophenyl) thiosulfonic acid; and making bis(4-dimethylaminophenyl) thiosulfonic acid carry out ring-closing reactions to obtain methylene blue. The provided preparation method has the advantages of high product purity, simple technology flow, low manufacture cost, suitability for industrial production, easily-available raw materials, and little pollution to the environment.
Owner:JIANGSU HERUN PHARMA CO LTD
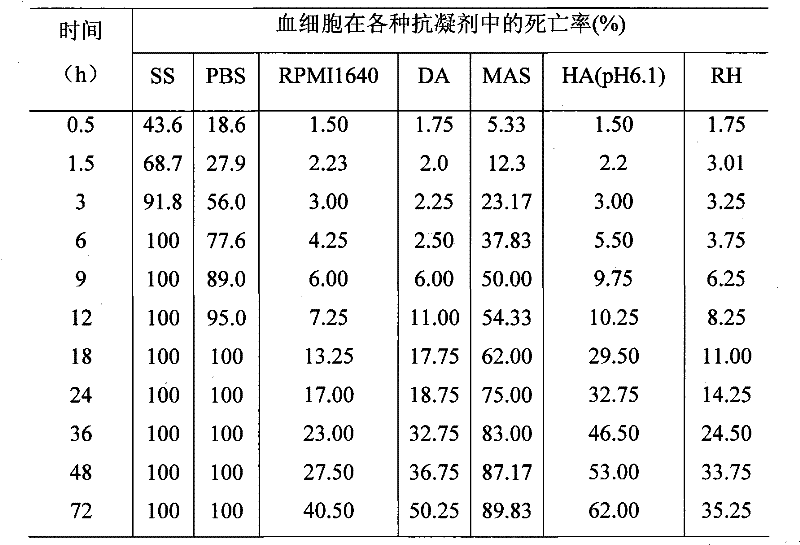
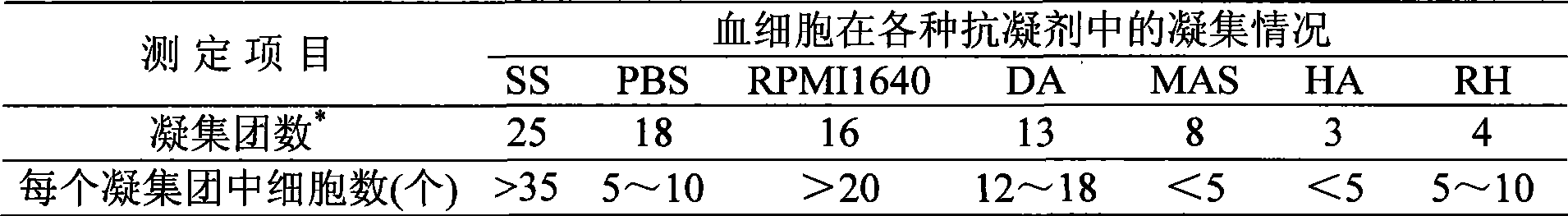
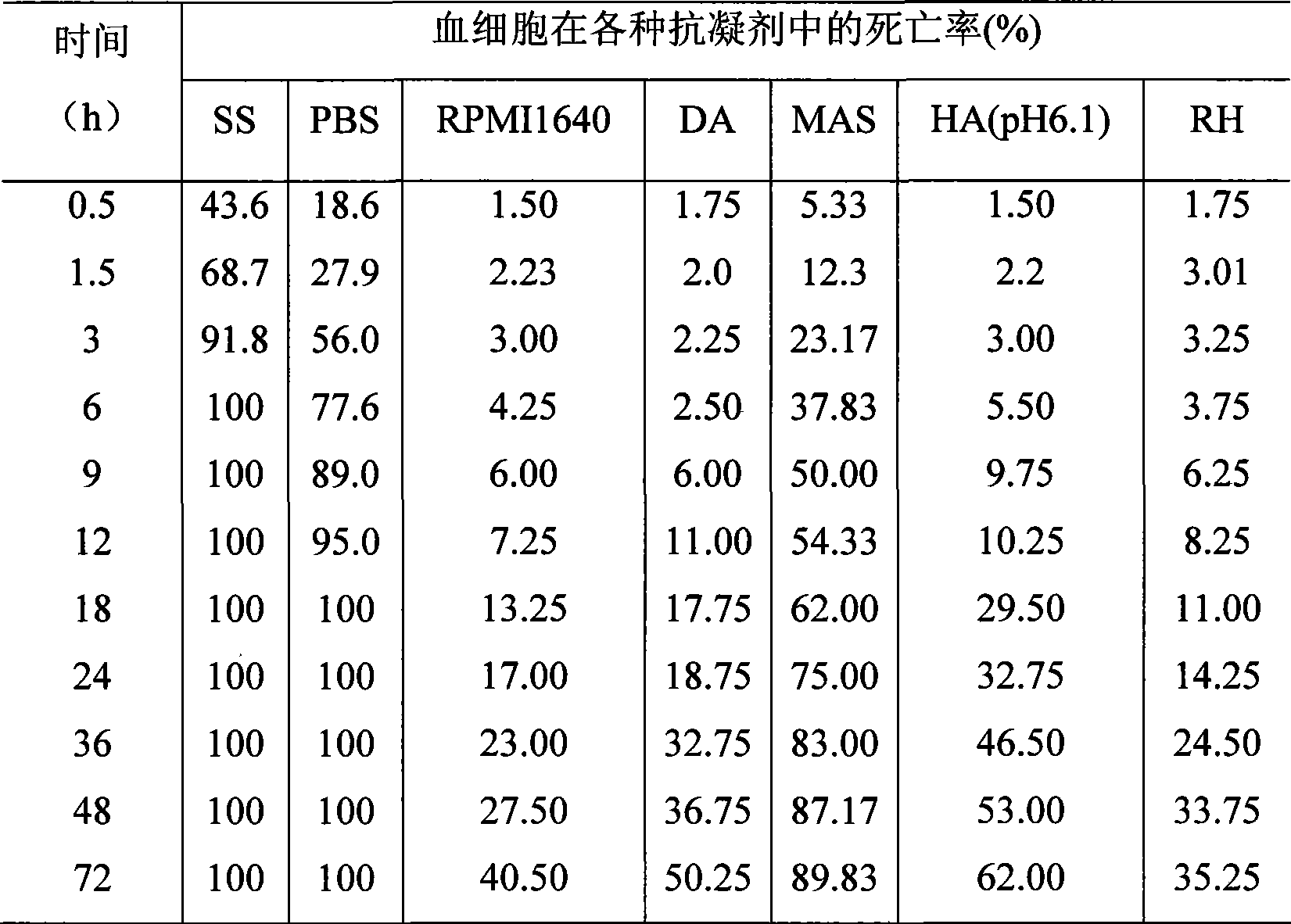
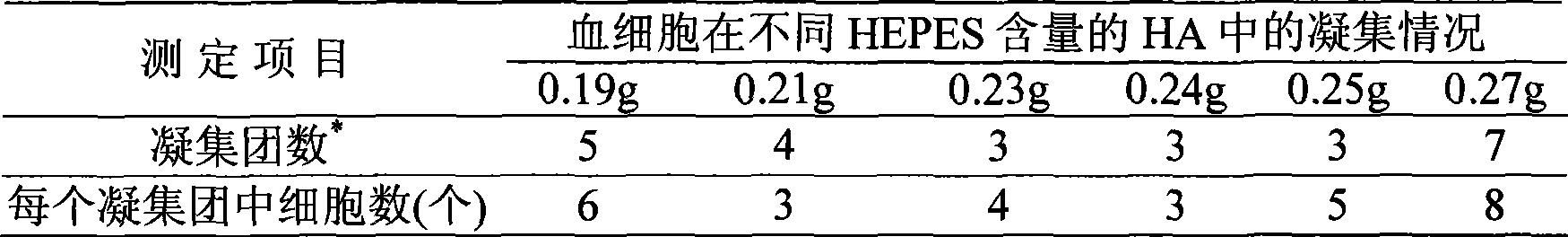
Abalone blood cell anticoagulation protecting agent and preparation method thereof
The invention discloses an abalone blood cell anticoagulant protective agent and a preparation method thereof, and relates to an anticoagulant protective agent. The invention provides an abalone blood cell anticoagulant protective agent for maintaining the activity of isolated abalone blood cells within longer time and a preparation method thereof. The abalone blood cell anticoagulant protective agent comprises the following raw materials by mass percentage: 1.95 to 2.08 percent of glucose, 0.78 to 0.8 percent of sodium citrate (2H2O), 0.063 to 0.107 percent of citric acid, 0.37 to 0.57 percent of sodium chloride, 0.21 to 0.24 percent of hydroxyethyl piperazinyl ethyl thiosulfonic acid, and 100 percent of double distilled water, wherein the pH value is between 6.0 and 6.4. The abalone blood cell anticoagulant protective agent is obtained by dissolving the glucose, the sodium citrate (2H2O), the sodium chloride and the hydroxyethyl piperazinyl ethyl thiosulfonic acid into the double distilled water, stirring and dissolving the mixture, using the citric acid to adjust the pH value to be between 6.0 and 6.4, and performing high-temperature sterilization.
Owner:XIAMEN UNIV
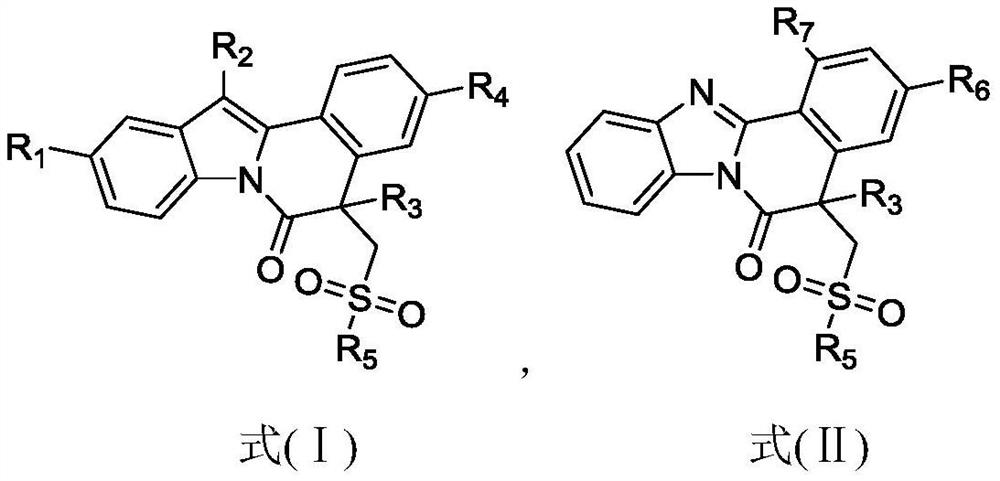
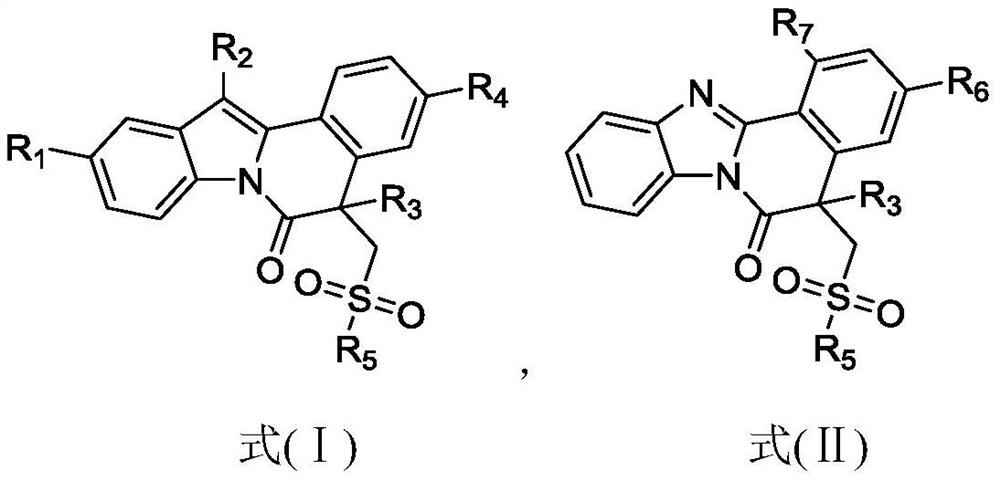
Isoquinoline compound as well as preparation method and application thereof
ActiveCN113698399AAntiproliferative activityThe reaction system is simple and safeOrganic chemistryAntineoplastic agentsBenzoic acidChemical synthesis
The invention discloses an isoquinoline compound as well as a preparation method and application thereof, and relates to the technical field of chemical synthesis. The isoquinoline compound is an indolo [2, 1-a] isoquinoline compound or a benzimidazo [2, 1-a] isoquinoline-6 (5H) ketone compound, and the preparation method comprises the following steps: dissolving a 1-(2, 3-diphenyl-1H-indole-1-yl)-2-methyl propenyl-1-ketone compound or a 1-methyl acryloyl-2-aryl-benzimidazole compound and an S-phenyl phenyl thiosulfonate compound in an organic solvent, adding tert-butyl benzoate oxide and a catalytic amount of copper bromide, and reacting at 110 DEG C to obtain the isoquinoline compound. The synthesis process does not need diazo compounds, has the characteristics of cheap and easily available raw materials, mild reaction conditions, simplicity and convenience in operation, high regioselectivity and high yield, and is beneficial to industrial production.
Owner:ZHEJIANG PROVINCIAL PEOPLES HOSPITAL
Methods of Chemical Synthesis and Purification of Diaminophenothiazinium Compounds Including Methylthioninium Chloride (Mtc)
This invention pertains generally to the field of chemical synthesis and purification, and more specifically to methods of synthesizing and purifying certain 3,7 diamino-phenothiazin-5-ium compounds (referred to herein as “diaminophenothiazinium compounds”) including Methylthioninium Chloride (MTC) (also known as Methylene Blue). In one embodiment, the method comprises the steps of, in order: nitrosylation (NOS); nitrosyl reduction (NR); thiosulfonic acid formation (TSAF); oxidative coupling (OC); Cr(VI) reduction (CR); isolation and purification of zwitterionic intermediate (IAPOZI); ring closure (RC); chloride salt formation (CSF); one of: sulphide treatment (ST); dimethyldithiocarbamate treatment (DT); carbonate treatment (CT); ethylenediaminetetraacetic acid treatment (EDTAT); organic extraction (OE); and recrystallisation (RX). The present invention also pertains to the resulting (high purity) compounds, compositions comprising them (e.g., tablets, capsules), and their use in methods of inactivating pathogens, and methods of medical treatment and diagnosis, etc., for example, for tauopathies, Alzheimer's disease (AD), skin cancer, melanoma, viral diseases, bacterial diseases, or protozoal diseases. Wherein: each of R1 and R9 is independently selected from: —H; C1-4 alkenyl; and halogenated C1-4alkyl; each of R3NA and R3NB is independently selected from: C1-4 alkyl; C2-4alkenyl; and halogenated C4-1 alkyl; each of R7NA and R7NB is independently selected from: C1-4 alkyl; C2-4alkenyl; and halogenated C1-4 alkyl; and X is one or more anionic counter ions to achieve electrical neutrality.
Owner:WISTA LAB LTD
High purity diaminophenothiazinium compounds including methylthioninium chloride (MTC)
This invention pertains generally to the field of chemical synthesis and purification, and more specifically to methods of synthesizing and purifying certain 3,7 diamino-phenothiazin-5-ium compounds (referred to herein as “diaminophenothiaziniumcompounds”) including Methylhioninium Chloride (MTC) (also known as Methylene Blue). In one embodiment, the method comprises the steps of, in order: nitrosylation (NOS); nitrosyl reduction (NR); thiosulfonic acid formation (TSAF); oxidative coupling (OC); Cr(VI) reduction (CR); isolation and purification of zwitterionic intermediate (IAPOZI); ring closure (RC); chloride salt-formation (CSF); one of: sulphide treatment (ST); dimethyldithiocarbamate treatment (DT); carbonate treatment (CT); ethylenediaminetetraacetic acid treatment (EDTAT); organic extraction (OE); and recrystallisation (RX). The present invention also pertains to the resulting (high purity) compounds, compositions comprising them (e.g., tablets, capsules), and their use in methods of inactivating pathogens, and methods of medical treatment and diagnosis, etc., for example, for tauopathies, Alzheimer's disease (AD), skin cancer, melanoma, viral diseases, bacterial diseases, or protozoal diseases.
Owner:WISTA LAB LTD
Sodium salt of [poly-(2,5-dihydroxyphenylene)]-4-thiosulphuric acid of linear structure as regulator of cell metabolism and production method thereof
InactiveUS6117970APromote formationProduce some attenuationOrganic chemistryHydroxy compound active ingredientsThiosulfonic AcidsPara-Benzoquinone
PCT No. PCT / RU97 / 00338 Sec. 371 Date Apr. 28, 1999 Sec. 102(e) Date Apr. 28, 1999 PCT Filed Oct. 28, 1997 PCT Pub. No. WO98 / 18758 PCT Pub. Date May 7, 1998Sodium salt of (poly-(2,5-dihydroxy-phenylene))-4-thiosulfonic acid, being a cyclo-linear polymer, is proposed as the regulator of a cell metabolism. There are provided for the production of the claimed preparation: interaction of para-benzoquinone and sodium thiosulfate at the molar ratio of 10:1 to 2:1, separation of a final product, and purification from admixtures. The interaction of para-bernzoquinone and sodium thiosulfate is made in a water-organic medium, preferably in the water-acetone one, at the temperature of 40 to 70 C.
Owner:KORPORATSIAOLIPHEN ZAKRYTOE AKTSIONERNOE OBSCHESTVO
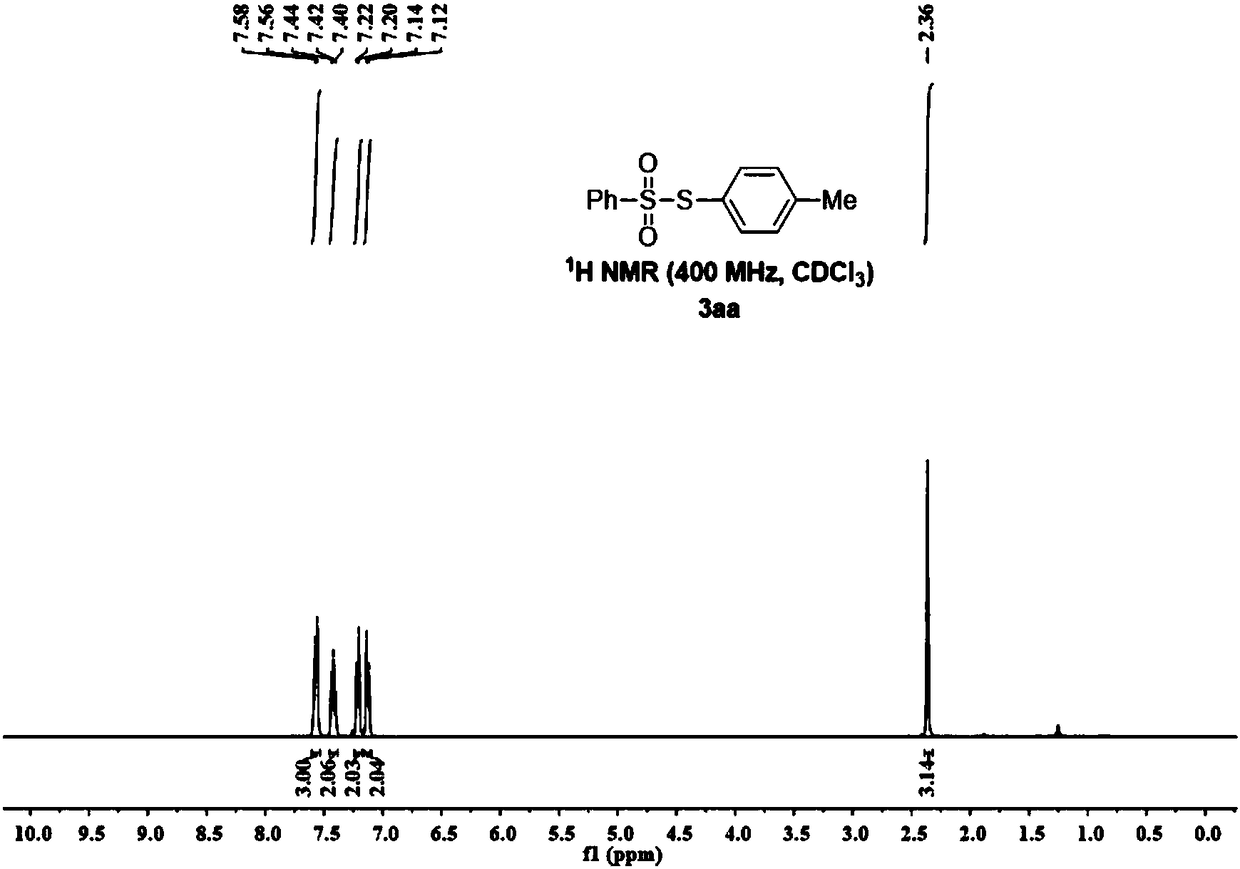
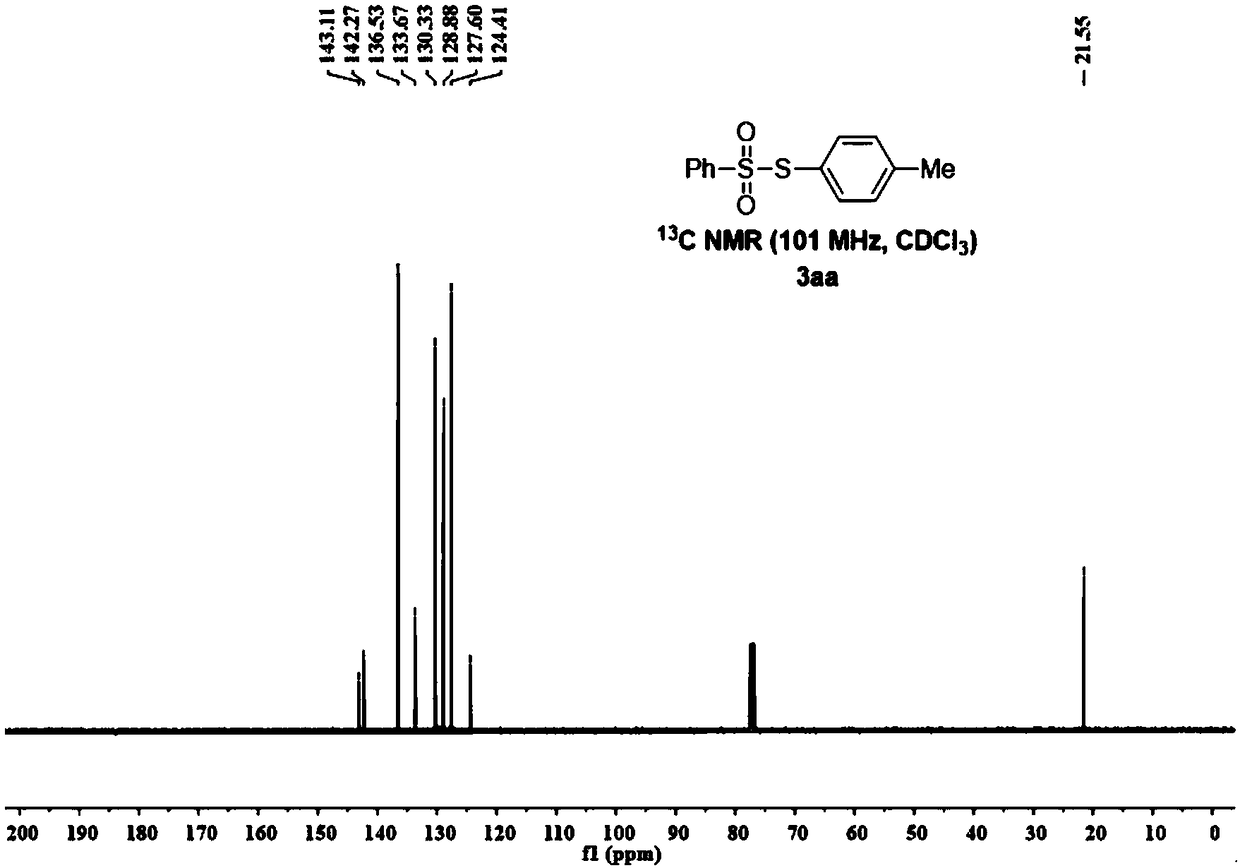
Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound
ActiveCN112442002AHigh industrial practical valueSimple and fast operationOrganic chemistryThiosulfonic AcidsThio-
The invention discloses a method for synthesizing a 11-sulfenylnaphtho[2,3-b]benzofuran compound. The method is characterized in that 2-ethyleneoxyphenylacetylene and thiosulfonate are used as substrates, disulfide is used as an additive, Na2-Eosin Y is used as a photosensitizer, and light-irradiation stirring is performed under inert gas and heating conditions to obtain the product 11-sulfenylnaphtho[2,3-b]benzofuran. The method has the advantages of no need for transition metal addition in reactions, simple operation, considerable yield, environmental protection performance and good application prospects.
Owner:GUILIN MEDICAL UNIVERSITY
Efficient pesticide for preventing and treating crop pests
The invention discloses an efficient pesticide for preventing and treating crop pests. The efficient pesticide is prepared from the following raw materials in parts by weight: 3-8 parts of dinitrobenzene, 4-6 parts of mineral oil, 5-9 parts of zosterin, 6-10 parts of epoxy resin, 4-8 parts of hexahydrophthalic anhydride, 1-5 parts of light calcium carbonate, 2-6 parts of chlorpyrifos, 0.5-2 parts of a stabilizing agent, 1.5-4 parts of a synergistic agent, 2-5 parts of acetyl methyl ammonium phosphate, 8-10 parts of ethion and 1-3 parts of sodium thiosulfonate. The efficient pesticide disclosed by the invention has the beneficial effects that the efficient pesticide for preventing and treating crop pests adopts a low-toxicity pesticide, and is low in toxicity and residue and can be used for effectively protecting the environment and crop quality under the condition of meeting the requirements of killing diseases and insect pests.
Owner:QINGDAO BAOLIKANG NEW MATERIALS
Preparation method of thiosulfonate compound
ActiveCN112812046ARaw materials are easy to obtainThe reaction is easy to operateOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystOrganic synthesis
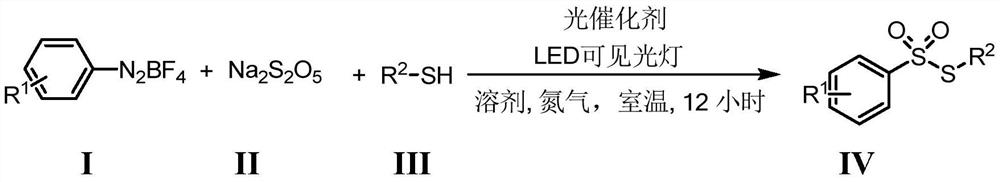
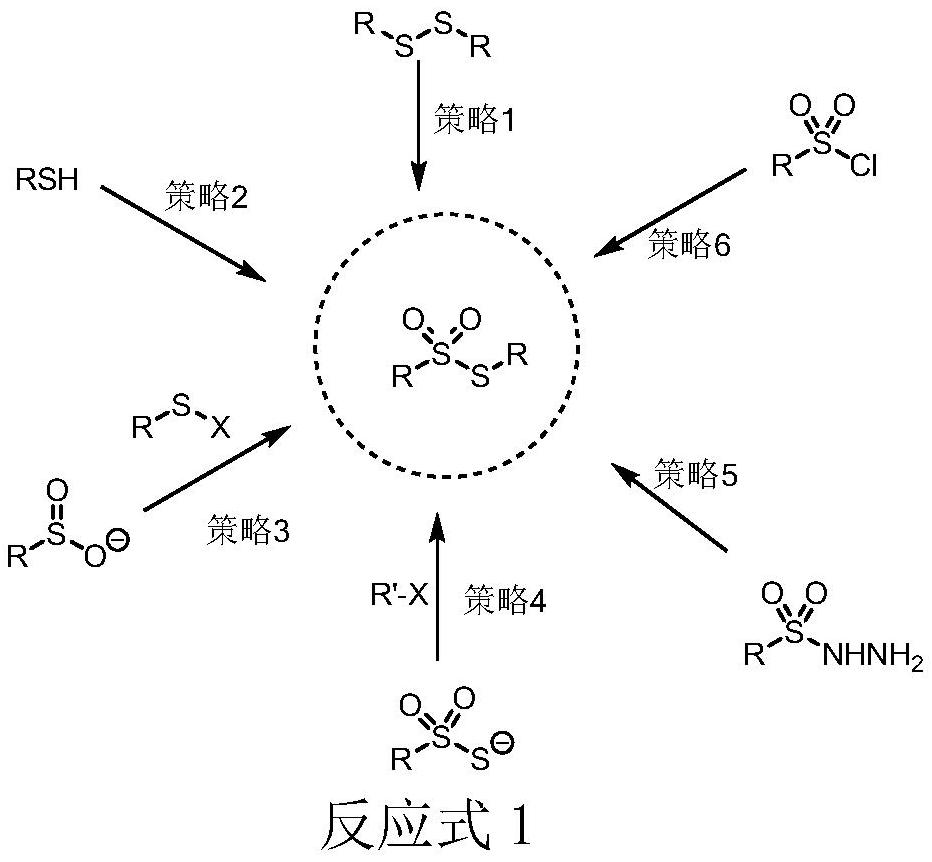
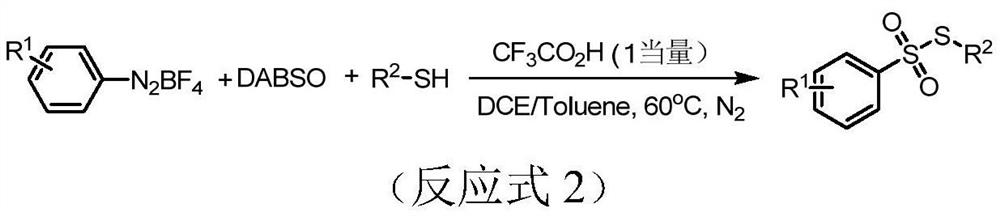
The invention belongs to the field of organic synthetic chemistry, and particularly discloses a preparation method of a thiosulfonate compound. The specific preparation process comprises the following steps: taking aryl diazonium salt, sodium pyrosulfite and thiophenol / mercaptan as raw materials, taking organic dye as a photocatalyst, adding an organic solvent, and reacting for 12 hours at room temperature under the irradiation of an LED visible light lamp and nitrogen protection to generate thiosulfonate; and after the reaction is finished, adding distilled water into the reaction system, extracting the reaction liquid with ethyl acetate, concentrating the extraction liquid to obtain a crude product, and carrying out silica gel column chromatography separation on the crude product to obtain thiosulfonate. According to the method, simple and easily available sodium pyrosulfite is used as a sulfone source, cheap organic dye is used as a photocatalyst, clean light energy is used as a reaction energy source, a high-energy-consumption heating device and a metal catalyst or strong acid are not used, the reaction condition is mild, the operation is simple and convenient, and a green synthesis strategy is provided for thiosulfonate.
Owner:CHINA ACAD OF SCI NORTHWEST HIGHLAND BIOLOGY INST +1
Abalone blood cell anticoagulation protecting agent and preparation method thereof
The invention discloses an abalone blood cell anticoagulant protective agent and a preparation method thereof, and relates to an anticoagulant protective agent. The invention provides an abalone blood cell anticoagulant protective agent for maintaining the activity of isolated abalone blood cells within longer time and a preparation method thereof. The abalone blood cell anticoagulant protective agent comprises the following raw materials by mass percentage: 1.95 to 2.08 percent of glucose, 0.78 to 0.8 percent of sodium citrate (2H2O), 0.063 to 0.107 percent of citric acid, 0.37 to 0.57 percent of sodium chloride, 0.21 to 0.24 percent of hydroxyethyl piperazinyl ethyl thiosulfonic acid, and 100 percent of double distilled water, wherein the pH value is between 6.0 and 6.4. The abalone blood cell anticoagulant protective agent is obtained by dissolving the glucose, the sodium citrate (2H2O), the sodium chloride and the hydroxyethyl piperazinyl ethyl thiosulfonic acid into the double distilled water, stirring and dissolving the mixture, using the citric acid to adjust the pH value to be between 6.0 and 6.4, and performing high-temperature sterilization.
Owner:XIAMEN UNIV
Fipronil-containing composite pesticide
A pipronil-containing composite pesticide is prepared from the following raw materials, by weight, 6-11 parts of fipronil, 3-8 parts of sodium thiosulfonate, 2-6 parts of turpentine, 1-5 parts of fermented milk, 4-7 parts of melamine, 2-4 parts of a binder, 6-10 parts of dodecyl dimethyl benzyl ammonium chloride, 3-6 parts of campesterol, 1-5 parts of anhydrous ethanol, 2-5 parts of trifluoro(methanol)boron, 1-3 parts of methyl amyl carbinol, 2-6 parts of acephate and 1-4 parts of xylene. The pipronil-containing composite pesticide can be cooperatively used with other chemical reagents, has small toxicity, can quickly kill most insects, and can be used for a long term.
Owner:QINGDAO TORIX ELECTRONICS TECH
A kind of preparation method of thiosulfonate compound in aqueous phase
ActiveCN109796391BRealize free radical coupling reactionSimple and fast operationOrganic chemistryAir atmosphereSulfohydrazide
The invention relates to a preparation method of thiosulfonate compounds in water phase. In the method, sulfonyl hydrazide, accelerator, oxidizing agent and solvent water are added into a Schlenk reaction bottle, stirred and reacted under certain temperature and air atmosphere conditions, and thiosulfonate compounds are obtained through free radical coupling reaction.
Owner:NINGBO UNIV
Methods of chemical synthesis and purification of diaminophenothiazinium compounds including methylthioninium chloride (MTC)
This invention pertains generally to the field of chemical synthesis and purification, and more specifically to methods of synthesizing and purifying certain 3,7 diamino-phenothiazin-5-ium compounds (referred to herein as “diaminophenothiaziniumcompounds”) including Methylhioninium Chloride (MTC) (also known as Methylene Blue). In one embodiment, the method comprises the steps of, in order: nitrosylation (NOS); nitrosyl reduction (NR); thiosulfonic acid formation (TSAF); oxidative coupling (OC); Cr(VI) reduction (CR); isolation and purification of zwitterionic intermediate (IAPOZI); ring closure (RC); chloride salt-formation (CSF); one of: sulphide treatment (ST); dimethyldithiocarbamate treatment (DT); carbonate treatment (CT); ethylenediaminetetraacetic acid treatment (EDTAT); organic extraction (OE); and recrystallisation (RX). The present invention also pertains to the resulting (high purity) compounds, compositions comprising them (e.g., tablets, capsules), and their use in methods of inactivating pathogens, and methods of medical treatment and diagnosis, etc., for example, for tauopathies, Alzheimer's disease (AD), skin cancer, melanoma, viral diseases, bacterial diseases, or protozoal diseases.
Owner:WISTA LAB LTD
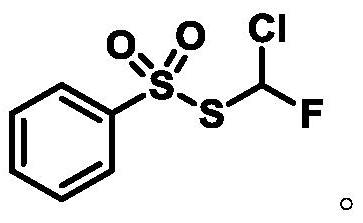
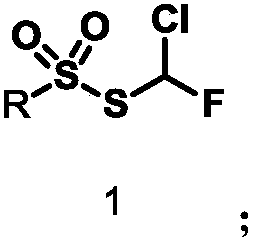
A compound containing monofluorochloromethylthiosulfonate, its preparation method and application
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Methods of chemical synthesis of diaminophenothiazinium compounds involving the use of persulfate oxidants
Described are methods of synthesizing and purifying certain 3,7-diamino-phenothiazin-5-ium compounds (referred to herein as “diaminophenothiazinium compounds”) including Methylhioninium Chloride (MTC) (also known as Methylene Blue). In one embodiment, the method comprises the steps of, in order: a thiosulfonic acid formation (TSAF); an oxidative coupling (OC); and a ring closure (RC). Also described are resulting compounds and compositions comprising them (e.g., tablets, capsules) for use in methods of medical treatment and diagnosis, etc., for example, for tauopathies, or Alzheimer's disease (AD).
Owner:WISTA LAB LTD
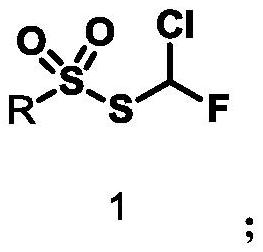
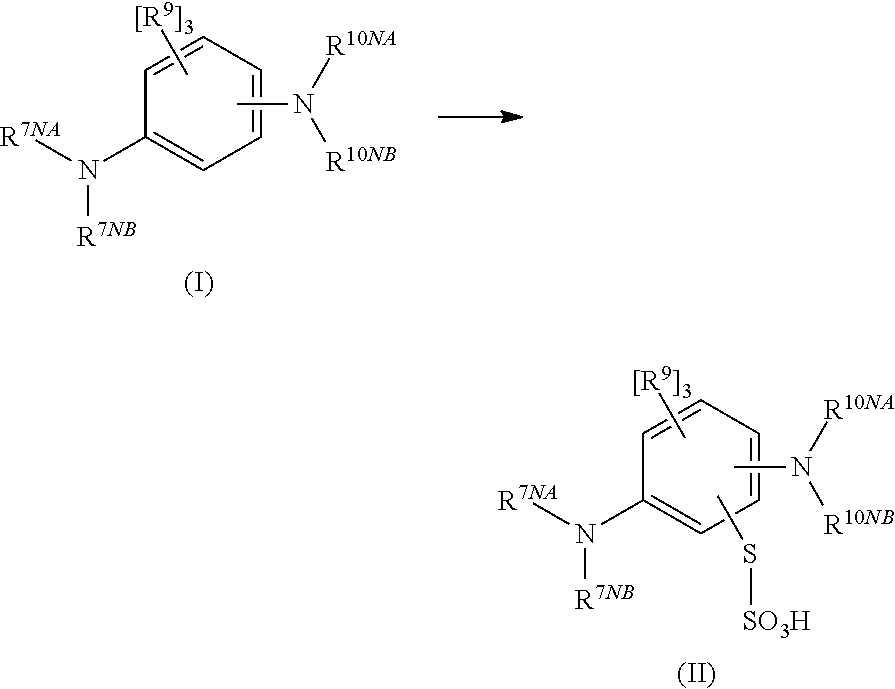
Synthesis of a thiosulfonic acid by a step of periodate mediated oxidative coupling of a thiosulfonic acid with an aniline
The present invention pertains generally to the field of chemical synthesis, and more particularly to methods for the chemical synthesis of a thiosulfonic acid of Formula (1) by a step of periodate mediated oxidative coupling of a thiosulfonic acid of Formula (2) with an aniline of Formula (3), as described herein. The present invention also relates to such methods which incorporate one or more additional (subsequent and / or preceding) steps, for example, to prepare compounds of Formula (5) from compounds of Formula (1); to prepare compounds of Formula (6) from compounds of Formula (5); and to prepare compounds of Formula (2) from compounds of Formula (4), as described herein.
Owner:WISTA LAB LTD
Process for manufacture of in vivo stain composition
InactiveUS20010007904A1Organic chemistryLuminescence/biological staining preparationThiosulfonic AcidsIn vivo
A process for synthesizing 2-amino-5-dimethlyaminophenyl thiosulfonic acid comprises the step of oxidizing N,N'-dimethyl-rho-phenylene-diamine in the presence of a source of thiosulfate ions, while maintaining the temperature of the reaction mixture not higher than about 10° C. This compound is useful as an intermediate in the synthesis of toluidine blue O. A process for manufacturing toluidine blue O with improved yield, includes the step of preparing the intermediate 2-amino-5-diethylaminopropyl thiosulfonic acid according to the above described procedure.
Owner:DEN MAT HLDG
Synthesis method of asymmetric thiosulfonic acid compound
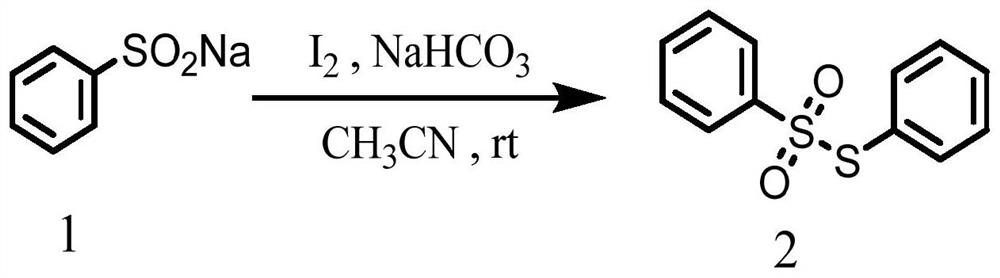
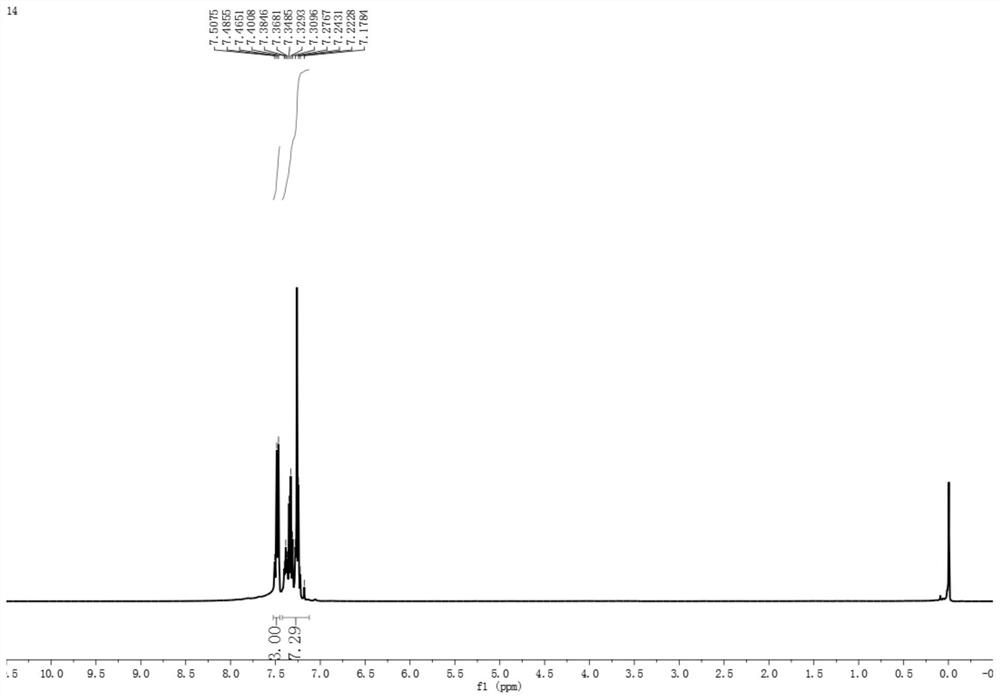
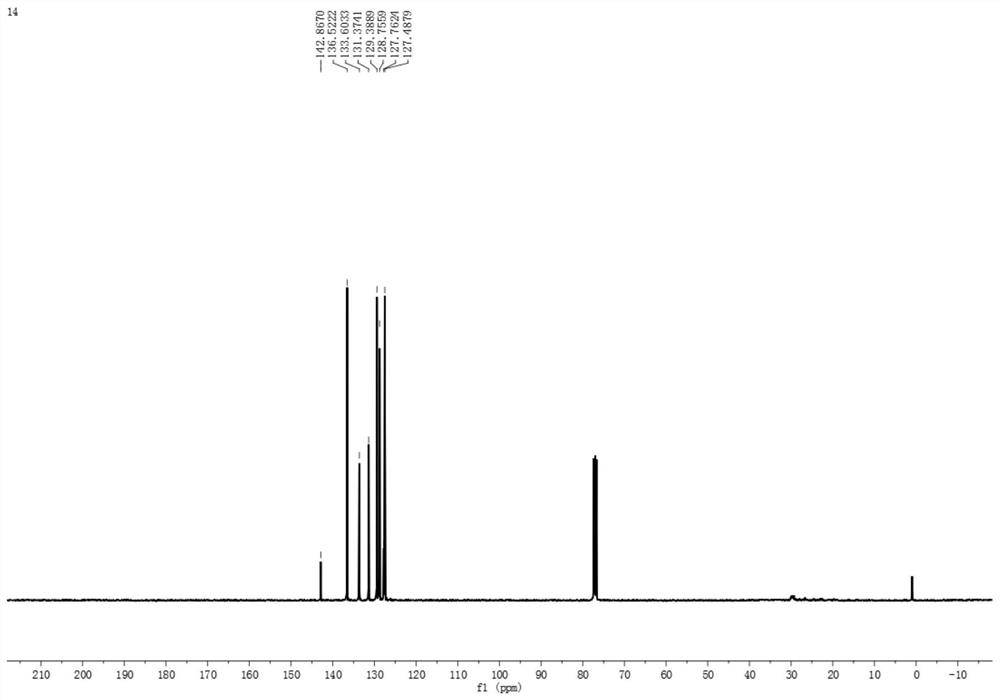
PendingCN114874115AReduce use costSimple reaction conditionsOrganic chemistryChemical recyclingSodium bicarbonatePtru catalyst
The invention discloses a synthesis method of an asymmetric thiosulfonic acid compound, belongs to the field of organic synthesis, and mainly solves the problems of great environmental damage, high cost and the like in the prior art. The synthesis method specifically comprises the following steps: (1) dissolving sodium benzenesulfinate in an acetonitrile solvent; (2) adding sodium bicarbonate and iodine as catalysts for catalytic reaction, and cooling; and (3) evaporating to remove the solvent under reduced pressure, and purifying. According to the method, the use cost of a catalyst in an existing thiosulfonic acid compound synthesis method is remarkably reduced, reaction conditions are greatly simplified, and the technical problems that in a traditional method, raw materials are high in price, synthesis steps are tedious, and treatment is difficult after synthesis are solved; the asymmetric thiosulfonic acid compound is prepared by catalyzing sodium benzenesulfinate under the condition that iodine and sodium bicarbonate serve as catalysts, raw materials are easy to obtain and low in price, reaction conditions are mild, aftertreatment is simple, and the yield of the obtained target product is high.
Owner:YULIN NORMAL UNIVERSITY
Pesticide composition containing thiosulfonic acid ester fungicide
The invention discloses a pesticide composition containing a thiosulfonic acid ester fungicide. The pesticide composition contains 10-60% of thiosulfonic acid ester, powders of 1-40 parts of methoxy-crylic acid fungicide, a soluble concentrate, a microemulsion, an emulsion in water, a suspending agent or missible oil. The pesticide composition is a compound composition of the thiosulfonic acid ester fungicide and the methoxy-crylic acid fungicide, high synergetic effect can be generated, and the capabilities of sterilization, disease prevention and healing can be reinforced, so that the crop can be prevented from falling ill in the growth process, and the yield and the quality are improved; the pesticide composition has a broad-spectrum sterilization capability, can prevent the diseases caused by various germs, and particularly plays the role of forcefully killing the moulds; and according to the pesticide composition, the use dose is reduced relatively, and the using cost is lowered relatively; the pesticide composition is processed to be a water-based environment-friendly agent, such as the emulsion in water, the microemulsion, a suspending agent and a suspension emulsion, the use dosage of the organic solvent is reduced, and the safety of the pesticide composition is improved.
Owner:夏建中
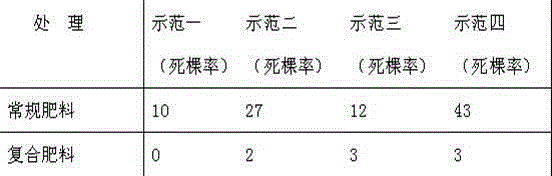
A compound fertilizer for preventing and treating dead vegetables and its preparation method
ActiveCN103483054BPreventing and Treating Dead Tree ProblemsGood effectFertilizer mixturesBiotechnologyBacillus licheniformis
The invention relates to the field of agricultural fertilizers, in particular to a compound fertilizer for preventing and treating dead vegetables and a preparation method thereof. Mix 500,000 parts of sodium humate, 100,000 parts of urea, 200,000 parts of avermectin powder, and 100,000 parts of lemon pomace in a weight ratio, grind them to more than 80 meshes, enter the stirring tank, and adjust the pH value to 7.6; add 1,000,000 parts of fertilizer during the stirring process 2000 parts of Bacillus licheniformis, 2000 parts of Bacillus subtilis, 1000 parts of sodium naphthalene acetate, 800 parts of gertyl milk powder, 200 parts of gibberellic acid, 1000 parts of iron powder, 1000 parts of zinc powder, 1000 parts of copper powder, 1000 parts of boron powder, 20 parts of N-(2-benzoimidazolyl)-methyl carbamate, 15 parts of 3-hydroxy-5-methylisoxazole, 10 parts of tolclofos-methyl, ethanethiosulfonate 20 parts of ethyl acetate. The effect is obvious, and the problem of dead vegetables can be effectively prevented and treated.
Owner:SHANDONG GOOD BROS FERTILIZER CO LTD
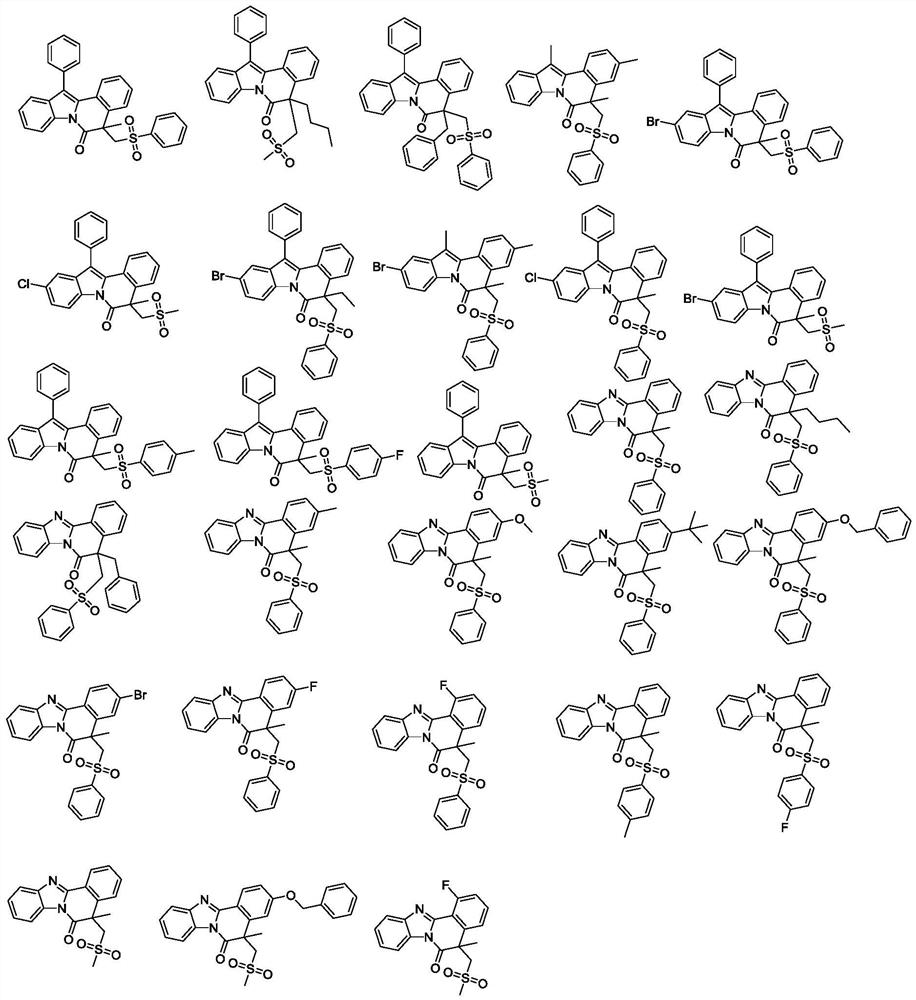
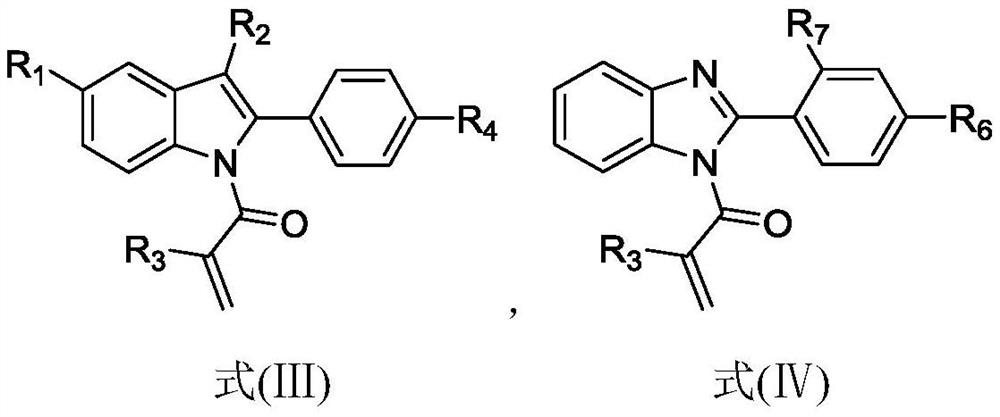
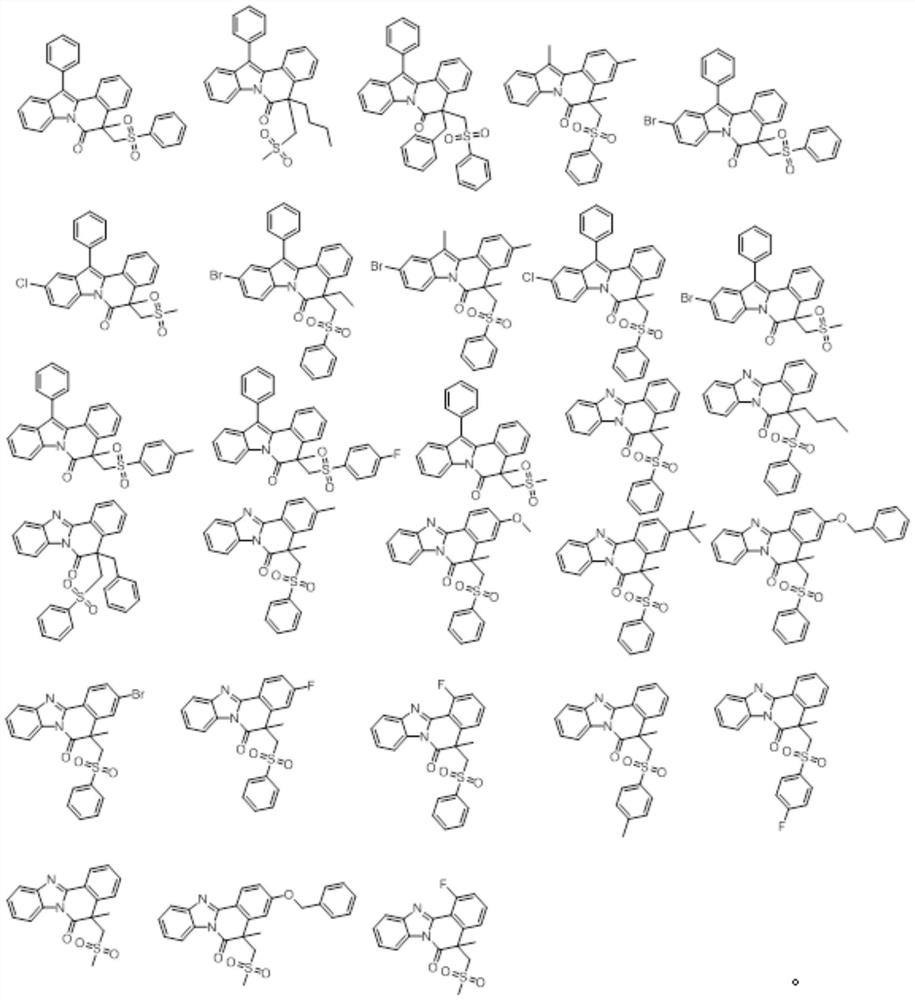
A kind of isoquinoline compound and its preparation method and application
ActiveCN113698399BAntiproliferative activityThe reaction system is simple and safeOrganic chemistryAntineoplastic agentsBenzoic acidChemical synthesis
The invention discloses an isoquinoline compound, a preparation method and application thereof, and relates to the technical field of chemical synthesis. The isoquinoline compound is an indolo[2,1-a]isoquinoline compound or a benzimidazolo[2,1-a]isoquinolin-6(5H)ketone compound, and the preparation method is as follows: 1‑(2,3‑diphenyl‑1H‑indol‑1‑yl)‑2‑methacryl‑1‑ketone compound or 1‑methacryl‑2‑aryl‑benzimidazole The compounds and S-phenylbenzenethiosulfonate compounds are dissolved in an organic solvent, tert-butyl benzoate and a catalytic amount of copper bromide are added to react at 110°C to obtain isoquinoline compounds. The synthesis process of the invention does not require the participation of diazonium compounds, has the characteristics of cheap and easy-to-obtain raw materials, mild reaction conditions, simple operation, high regioselectivity and high yield, and is beneficial to industrial production.
Owner:ZHEJIANG PROVINCIAL PEOPLES HOSPITAL
A kind of synthetic method of preparing thiosulfonate based on disproportionation reaction of sodium sulfinic acid
ActiveCN108586302BEasy to operateRaw materials are easy to getOrganic compound preparationSulfonic acid amide preparationThiosulfinatePtru catalyst
The invention relates to a synthetic method for preparing thiosulfonates on the basis of a sulfinic acid sodium salt disproportionated reaction. The preparation method comprises the steps that sulfinic acid sodium salts serve as the raw materials, boron trifluoride diethyl etherate serves as accelerant, dichloromethane serves as solvent, and by means of a disproportionation coupled reaction, a thiosulfinate compound with good biological activity is synthesized; two different sulfinic acid sodium salts serve as the raw materials, and by means of a crossed disproportionation coupled reaction method, an asymmetric thiosulfinate compound can be synthesized. By means of a one-pot method and a two-step method, and by means of a thiosulfonates intermediate prepared on site, a sulfones compound and a sulfonamides compound can be synthesized by directly starting from sulfinic acid sodium salts. Accordingly, a synthetic route for preparing thiosulfonates on the basis of the sulfinic acid sodiumsalt disproportionated reaction is provided for the first time, operation is easy, raw materials are easy to obtain, the condition is mild, no metal catalyst is needed, no additional oxidant or reductant is needed, and the prepared thiosulfonates can have the good biological activity, and can also serve as the intermediate of the reaction to be applied to organic synthesis.
Owner:SOUTH CHINA NORMAL UNIVERSITY
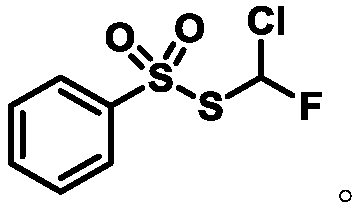
Monochlorofluoromethyl thiosulfonate containing compound, preparation method and application
The invention discloses a monochlorofluoromethyl thiosulfonate containing compound represented by a formula 1 shown in the description, a preparation method and an application. The preparation methodfor the compound represented by the formula 1 comprises the following step: subjecting a formula shown in the description to a reaction with sodium phenylsulfinate in a chlorine gas solution, therebyobtaining the compound. The compound represented by the formula 1 disclosed by the invention can serve as a monochlorofluoromethyl thiolation reagent and is high in application yield, thereby providing an important methodological research tool for the fields of medicines, pesticides and materials.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
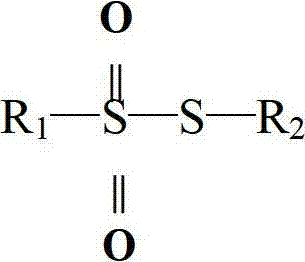

![Sodium salt of [poly-(2,5-dihydroxyphenylene)]-4-thiosulphuric acid of linear structure as regulator of cell metabolism and production method thereof Sodium salt of [poly-(2,5-dihydroxyphenylene)]-4-thiosulphuric acid of linear structure as regulator of cell metabolism and production method thereof](https://images-eureka.patsnap.com/patent_img/2d15603d-49f3-44c2-bd9b-45e633cee2ef/00000001_0000.png)
![Sodium salt of [poly-(2,5-dihydroxyphenylene)]-4-thiosulphuric acid of linear structure as regulator of cell metabolism and production method thereof Sodium salt of [poly-(2,5-dihydroxyphenylene)]-4-thiosulphuric acid of linear structure as regulator of cell metabolism and production method thereof](https://images-eureka.patsnap.com/patent_img/2d15603d-49f3-44c2-bd9b-45e633cee2ef/00000002_0000.png)
![Sodium salt of [poly-(2,5-dihydroxyphenylene)]-4-thiosulphuric acid of linear structure as regulator of cell metabolism and production method thereof Sodium salt of [poly-(2,5-dihydroxyphenylene)]-4-thiosulphuric acid of linear structure as regulator of cell metabolism and production method thereof](https://images-eureka.patsnap.com/patent_img/2d15603d-49f3-44c2-bd9b-45e633cee2ef/00000003_0000.png)
![Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound](https://images-eureka.patsnap.com/patent_img/1a559868-4712-4fad-a191-fda874e50aad/FDA0002823925220000011.png)
![Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound](https://images-eureka.patsnap.com/patent_img/1a559868-4712-4fad-a191-fda874e50aad/BDA0002823925230000011.png)
![Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound Method for synthesizing 11-sulfenylnaphtho[2,3-b]benzofuran compound](https://images-eureka.patsnap.com/patent_img/1a559868-4712-4fad-a191-fda874e50aad/BDA0002823925230000021.png)