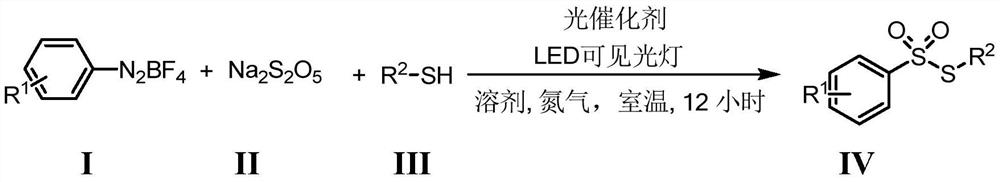
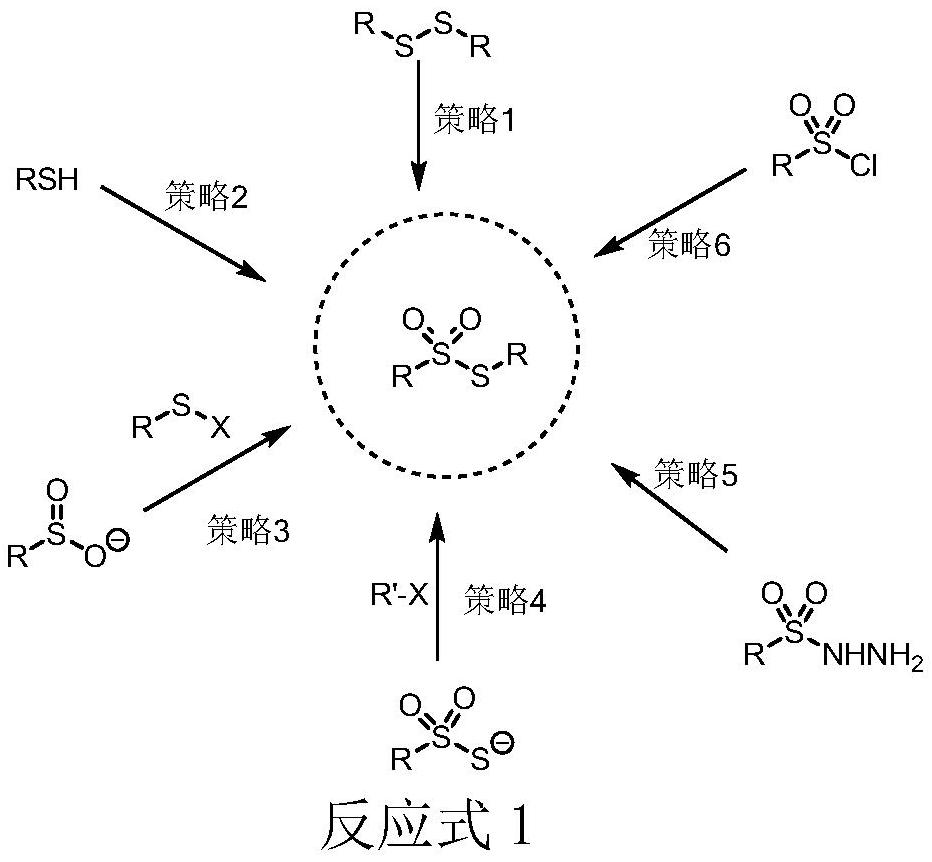
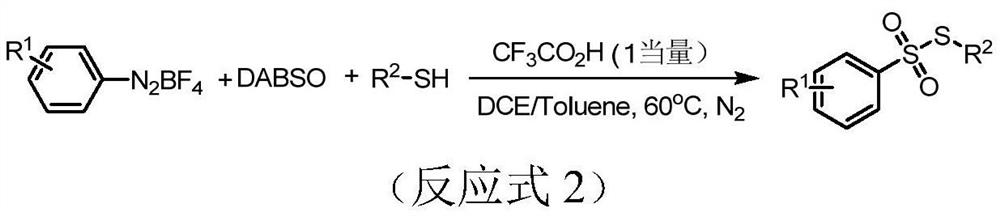
Preparation method of thiosulfonate compound
A technology of thiosulfonate and compound, which is applied in the field of preparation of photocatalytic thiosulfonate compound, which can solve the problems of a large amount of trifluoroacetic acid, high energy consumption, and corrosion of reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] At room temperature, add photocatalyst rhodamine 6G (0.002mmol), aryl diazonium salt 1a (0.4mmol), sodium metabisulfite 2 (0.4mmol), p-cresol 3a (0.2mmol), acetonitrile 2mL, mix well. Then, under the irradiation of a 3W white LED lamp, the reaction was carried out at room temperature under nitrogen protection for 12 hours. After the reaction was detected by TLC, distilled water was added, and then the reaction solution was extracted with ethyl acetate, and the extract was concentrated in vacuum (0.08Mpa) to no solvent to obtain the crude product, and then the petroleum ether with a volume ratio of 5:1 Washing with a mixed eluent of ethyl acetate, followed by flash column chromatography on a silica gel column, the thiosulfonate 4aa of this example was obtained with a yield of 75%.
[0034] The resulting product spectrum data is:
[0035] 1 H NMR (500MHz, CDCl 3 ): δ7.58-7.56(m,3H),7.42(dd,2H,J=7.9,7.8Hz),7.23(d,2H,J=8.1Hz),7.13(d,2H,J=8.0Hz) ,2.37(s,3H)....
Embodiment 2
[0037]
[0038] At room temperature, add photocatalyst water-soluble eosin (0.002mmol), aryl diazonium salt 1a (0.4mmol), sodium metabisulfite 2 (0.4mmol), p-cresol 3a (0.2mmol), acetonitrile 2mL, mix well. Then, under the irradiation of a 3W white LED lamp, the reaction was carried out at room temperature under nitrogen protection for 12 hours. After the reaction was detected by TLC, distilled water was added, and then the reaction solution was extracted with ethyl acetate, and the extract was concentrated in vacuum (0.08Mpa) to no solvent to obtain the crude product, and then the petroleum ether with a volume ratio of 5:1 Washing with a mixed eluent of ethyl acetate, followed by flash column chromatography on a silica gel column, the thiosulfonate 4aa of this example was obtained with a yield of 56%.
[0039] The resulting product spectrum data is:
[0040] 1 H NMR (500MHz, CDCl 3 ): δ7.58-7.56(m,3H),7.42(dd,2H,J=7.9,7.8Hz),7.23(d,2H,J=8.1Hz),7.13(d,2H,J=8.0Hz) ,2.37...
Embodiment 3
[0042]
[0043] At room temperature, the photocatalyst Rose Bengal B (0.002mmol), aryl diazonium salt 1a (0.4mmol), sodium metabisulfite 2 (0.4mmol), p-cresol 3a (0.2mmol), Acetonitrile 2mL, mix well. Then, under the irradiation of a 3W white LED lamp, the reaction was carried out at room temperature under nitrogen protection for 12 hours. After the reaction was detected by TLC, distilled water was added, and then the reaction solution was extracted with ethyl acetate, and the extract was concentrated in vacuum (0.08Mpa) to no solvent to obtain the crude product, and then the petroleum ether with a volume ratio of 5:1 Washing with a mixed eluent of ethyl acetate, followed by flash column chromatography on a silica gel column, the thiosulfonate 4aa of this example was obtained with a yield of 70%.
[0044] The resulting product spectrum data is:
[0045] 1 H NMR (500MHz, CDCl 3 ): δ7.58-7.56(m,3H),7.42(dd,2H,J=7.9,7.8Hz),7.23(d,2H,J=8.1Hz),7.13(d,2H,J=8.0Hz) ,2.37(s,3H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com