A kind of isoquinoline compound and its preparation method and application
A ketone compound and compound technology, which can be used in organic chemistry, drug combination, antitumor drugs, etc., can solve the problems of kinase mutation and drug effect decline, and achieve the effects of simple operation, simple and safe reaction system, and low reaction cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
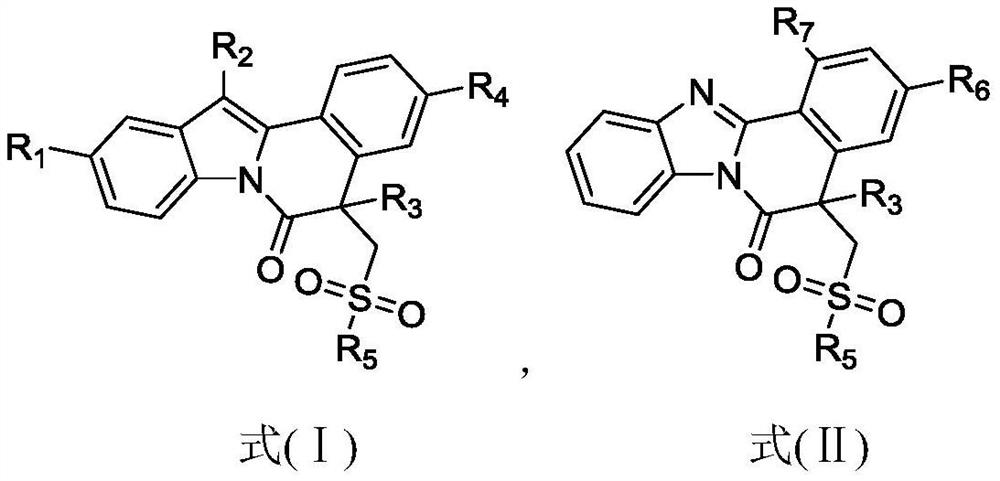
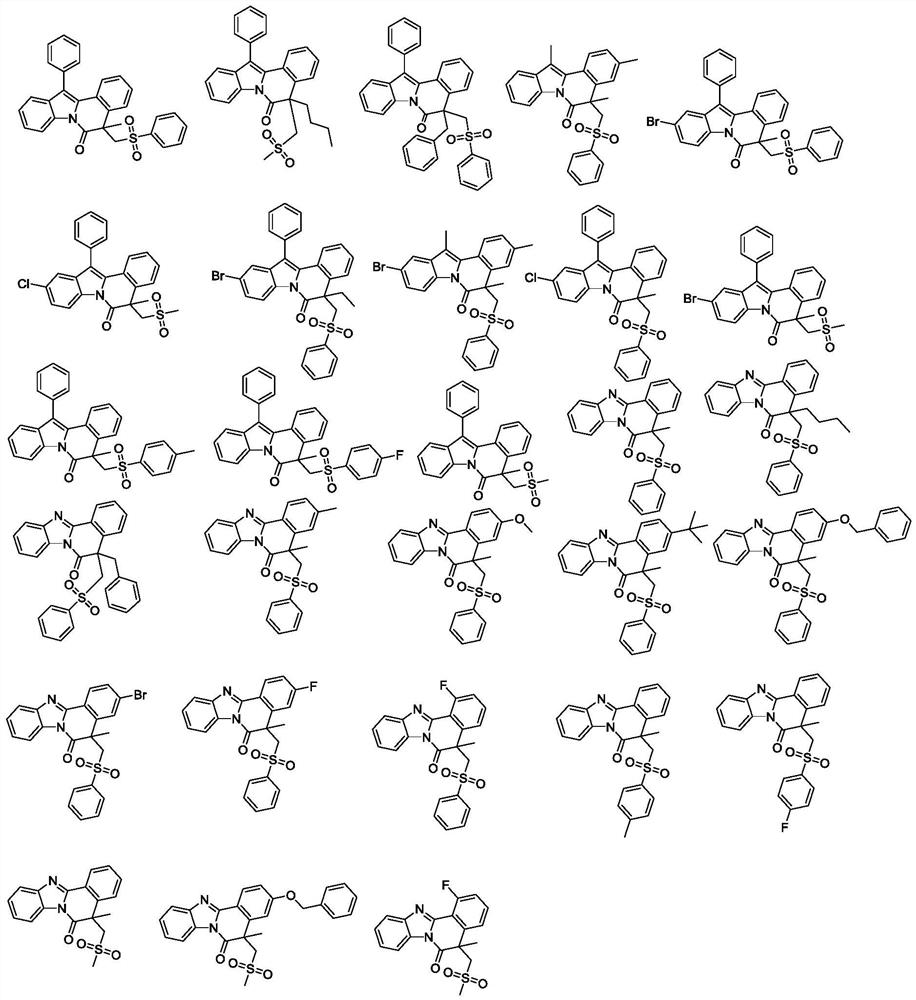
[0041] The preparation method of 5-methyl-12-phenyl-5-((phenylsulfo)methyl)indole[2,1-α]isoquinolin-6(5H)-one (1), the steps are as follows:
[0042] 1-(2,3-Diphenyl-1H-indol-1-yl)-2-methylpropenyl-1-one (0.1 mmol), S-phenylbenzenesulfonate (0.1 mmol), TBPB (tert-butyl peroxybenzoate) (0.3 mmol) and copper bromide (0.01 mmol) were dissolved in 3 mL of DCE (dichloroethylene), and the reaction was heated and stirred at 100 ° C for 10 hours. Quenching, extraction, drying, and distillation under reduced pressure to remove the solvent, and the residue was separated by silica gel column chromatography to obtain an oily substance.
[0043] The specific results are as follows:
[0044]
[0045] Yield: 85%. 1 H NMR (500MHz, CDCl 3 )δ8.49(d,J=8.2Hz,1H),7.53-7.41(m,6H),7.37(dd,J=8.1,1.1Hz,1H),7.34-7.28(m,2H),7.22-7.18 (m, 4H), 7.13 (dd, J=7.9, 0.8Hz, 1H), 6.99 (td, J=7.7, 1.3Hz, 1H), 6.95–6.85 (m, 1H), 4.52 (d, J=14.5 Hz,1H),3.90(d,J=16.3Hz,1H),1.61(s,3H). 13 C NMR (126MHz, CDCl...
Embodiment 2
[0047] The preparation method of 5-butyl-12-phenyl-5-((phenylsulfo)methyl)indole[2,1-α]isoquinolin-6(5H)-one (2), the steps are as follows:
[0048] 1-(2,3-Diphenyl-1H-indol-1-yl)-2-butylpropenyl-1-one (0.1 mmol), S-phenylbenzenesulfonate (0.1 mmol), TBPB (0.3 mmol) and copper bromide (0.01 mmol) were dissolved in 3 mL of DCE, and the reaction was heated and stirred at 100 ° C for 10 hours. After the reaction was completed, the solvent was quenched, extracted, dried, and distilled under reduced pressure to remove the solvent. Separation by silica gel column chromatography gave an oily substance.
[0049] The specific results are as follows:
[0050]
[0051] Yield: 35%. 1 H NMR (400MHz, CDCl 3 )δ8.58(d,J=8.2Hz,1H),7.63-7.48(m,7H),7.45(dd,J=8.0,1.0Hz,1H),7.43-7.37(m,1H),7.35-7.27 (m, 3H), 7.23 (t, J=7.7 Hz, 2H), 7.17–7.12 (m, 1H), 7.05 (td, J=7.6, 1.3 Hz, 1H), 7.01–6.96 (m, 1H), 4.57(d,J=14.6Hz,1H),3.92(d,J=14.6Hz,1H),2.34-2.11(m,1H),1.89-1.72(m,1H),1.19-1.00(m,2H) ,0....
Embodiment 3
[0053] The preparation method of 5-benzyl-12-phenyl-5-((phenylsulfo)methyl)indole[2,1-α]isoquinolin-6(5H)-one (3), the steps are as follows:
[0054] 1-(2,3-Diphenyl-1H-indol-1-yl)-2-benzylpropenyl-1-one (0.1 mmol), S-phenylbenzenesulfonate (0.1 mmol), TBPB (0.3 mmol) and copper bromide (0.01 mmol) were dissolved in 3 mL of DCE, and the reaction was heated and stirred at 100 ° C for 10 hours. After the reaction was completed, the solvent was quenched, extracted, dried, and distilled under reduced pressure to remove the solvent. Separation by silica gel column chromatography gave an oily substance.
[0055] The specific results are as follows:
[0056]
[0057] Yield: 46%. 1 H NMR (500MHz, CDCl 3 )δ8.53(d, J=8.2Hz, 1H), 7.58–7.52 (m, 2H), 7.44–7.34 (m, 4H), 7.33–7.29 (m, 1H), 7.25 (dd, J=14.9, 7.5Hz, 3H), 7.17–7.13 (m, 1H), 7.14–7.08 (m, 1H), 7.08–7.01 (m, 2H), 6.88 (ddd, J=11.2, 9.1, 4.2Hz, 2H), 6.69 (t,J=7.7Hz,2H),6.69(t,J=7.7Hz,2H),6.37(d,J=7.1Hz,2H),4.78(d,J=14.6Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com