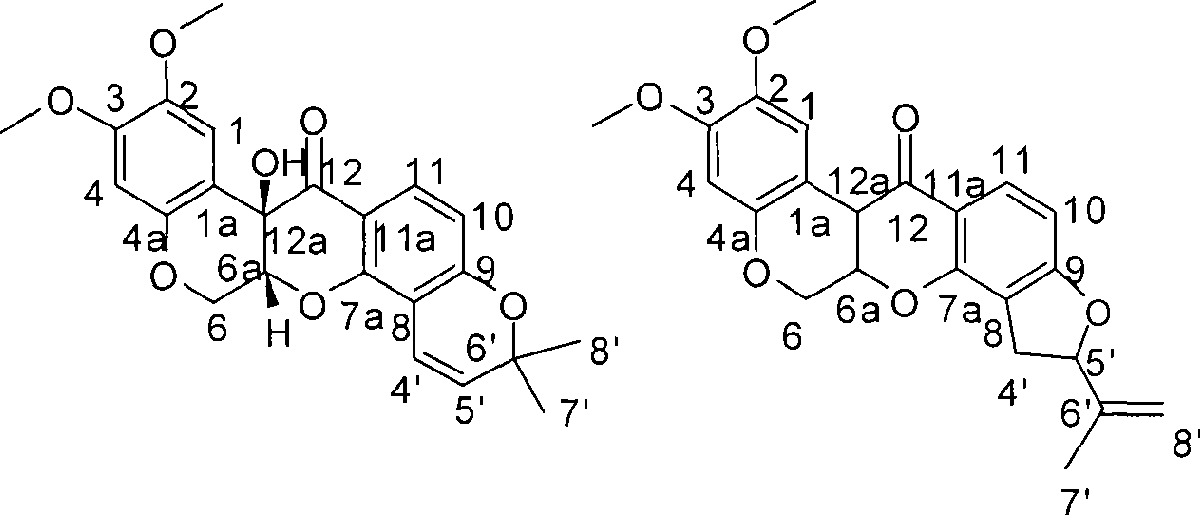
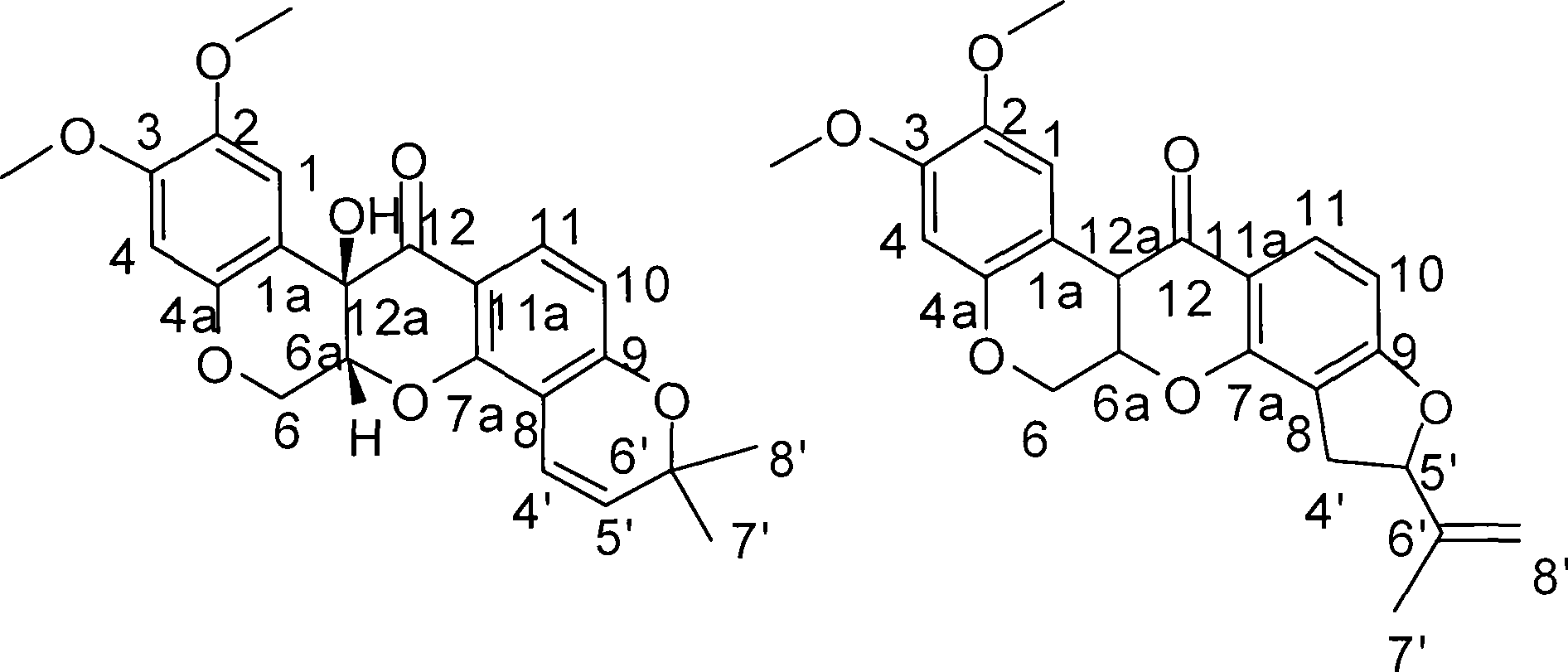
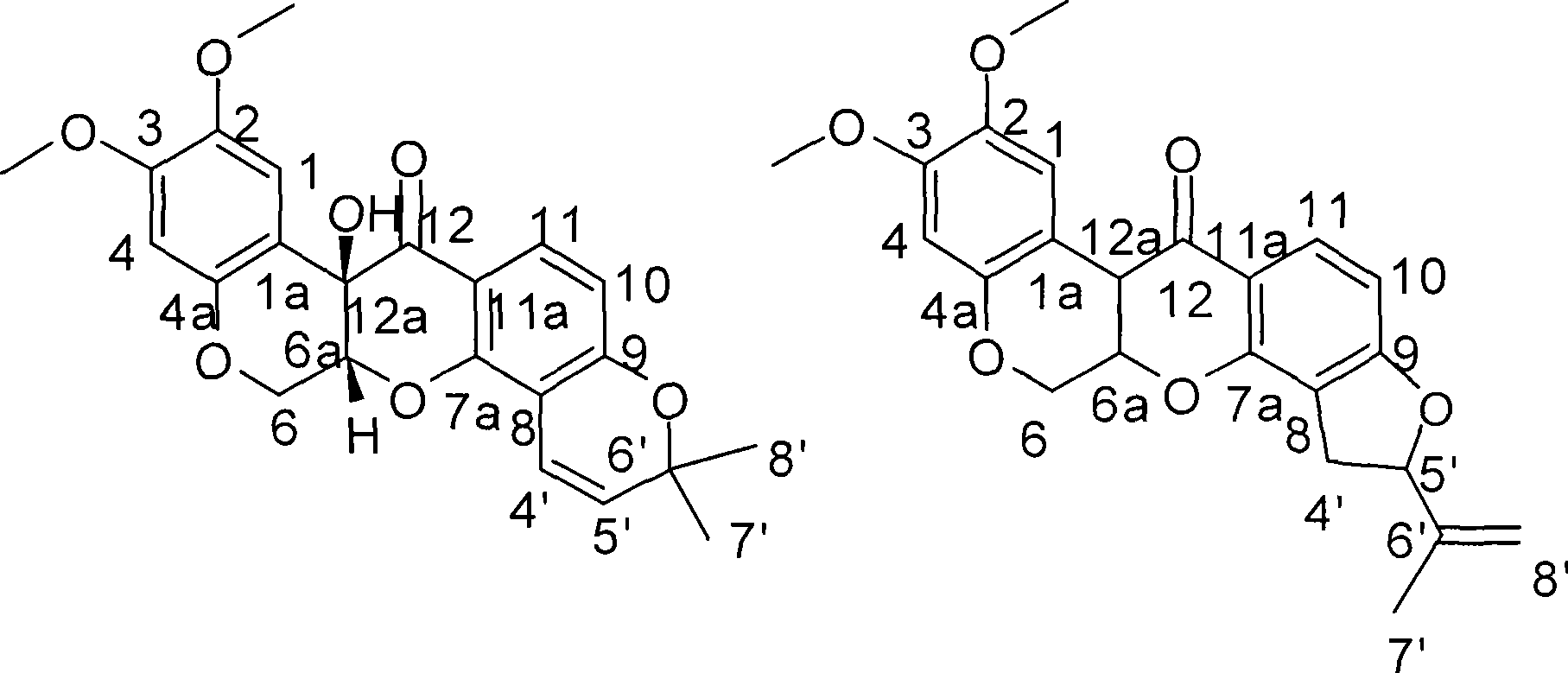
Method for preparing two isoflavones compounds and anti-tumor and anti-plant pathogen use thereof
A compound and use technology, applied in the field of use in the preparation of cell proliferation inhibitors or anti-tumor agents, can solve problems such as drugs that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1 Fermentative production and separation and purification of compound I II
[0015] 1 Fermentation production
[0016] Fermentation culture of production bacteria: According to the conventional method of cultivating microorganisms, take an appropriate amount of endophytic fungus YT2-02, inoculate it on the PDA solid slant medium, and cultivate it in a 28-degree Celsius incubator for 3 days.
[0017] Take an appropriate amount of endophytic fungus YT2-02 cultured on a slant for 3 days, and inoculate it into 120mL culture medium [Culture medium composition (g / L): maltose 20.0, mannitol 20.0, monosodium glutamate 10.0, KH 2 PO 4 0.5, MgSO 4 0.3, yeast extract 3.0, pH natural] in a 500mL Erlenmeyer flask, cultured on a shaker at 28°C and 120 rpm for 48 hours to obtain a seed culture solution of endophytic fungi. This seed culture solution is inoculated into the production culture solution after 200 milliliters of optimizations are housed by 10% inoculum size, m...
Embodiment 2
[0029] Example 2 Test of antitumor activity
[0030] 1 Experimental samples and experimental methods
[0031] Preparation of the test sample solution: the test sample is the pure compound I II isolated and refined in the above-mentioned Example 1. Accurately weigh an appropriate amount of sample and prepare a solution with the required concentration with DMSO for activity testing.
[0032] Cell lines and subculture of cells: HCT-8, A549, BEL-7402, BGC-823 and A2780 cell lines were used for activity testing. All kinds of cells were subcultured in RPMI-1640 medium containing 10% FBS in an incubator with 5% carbon dioxide at 37°C.
[0033] Cell Proliferation Inhibitory Activity Test Method (MTT Method)
[0034] The invention adopts the MTT method to test and evaluate the inhibitory activity of the tested sample on the proliferation of cancer cells. The dehydrogenase in the mitochondria of living cells can metabolize and reduce the yellow bromide 3-(4,5-dimethylthiazole)-2,5-d...
Embodiment 3
[0041] Example 3 Test of antitumor activity
[0042] In the present invention, the antifungal activity of the compound measured by the disc method is shown in Table 2, wherein rotenone has inhibitory activity on the growth of several plant pathogenic bacteria, and the inhibitory rate on grape white rot can reach 61.1%.
[0043] Table 2 Inhibitory effect of pure compounds on the growth of pathogen mycelium
[0044] Table2 Inhibition of compound against plant pathogen
[0045]
[0046] 4 Conclusion
[0047] Compounds I and II have anti-tumor effects on cancer cells derived from mammals including humans, compound I has a good inhibitory effect on grape rot fungi and apple rot fungi, and compound II has a good inhibitory effect on apple ringworm
[0048] Therefore, the compound III can be used as an anti-tumor agent (ie, an anti-tumor drug) in anti-tumor research, and has the potential to be developed as an anti-tumor drug; it can also be developed and applied as a biological p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com