Heteroaromatic ring compound with thiosemicarbazone structure unit and preparation method and application of compound
A technology of thiosemicarbazone and structural unit, which is applied in the directions of organic chemistry, drug combination, anti-tumor drugs, etc., can solve the problem of low inhibition effect on gastric cancer cell proliferation and so on.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
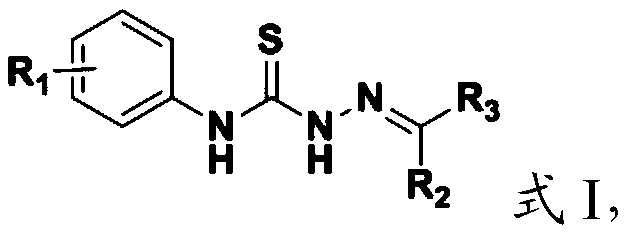
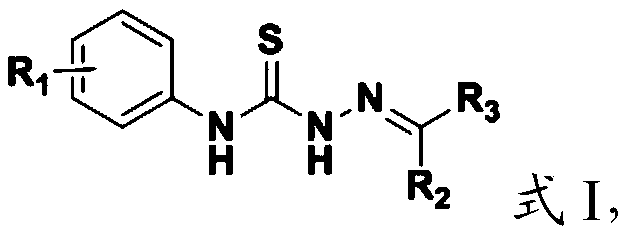
[0098] The present invention provides the preparation method of the aromatic heterocyclic compound containing the structural unit of thiosemicarbazone described in the above technical scheme, comprising the following steps:
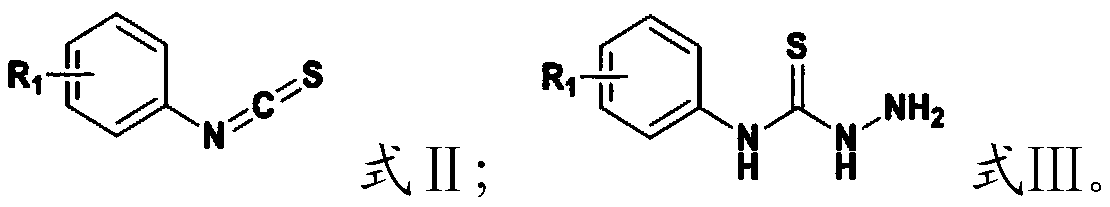
[0099] (1) Mix the compound having the structure shown in formula II, hydrazine hydrate and solvent I, and carry out a nucleophilic addition reaction to obtain the compound having the structure shown in formula III;
[0100] (2) the compound having the structure shown in formula III, R 2 -R 3Mixing with solvent II, and carrying out condensation reaction under the action of a catalyst, to obtain the aromatic heterocyclic compound containing the structural unit of thiosemicarbazone having the structure shown in formula I;
[0101]
[0102] In the present invention, unless otherwise specified, all raw materials are commercially available products well known to those skilled in the art.
[0103] In the present invention, the compound having the structure...
Embodiment 1
[0120] (1) At a stirring speed of 650rpm, the compound (1mmol) having the structure shown in Formula II-1 and 80wt% hydrazine hydrate aqueous solution (4mmol) were successively added to methanol (AR, 5mL) at a rate of 7° C. / min The heating rate was raised to 70°C, refluxed and stirred for 5h (TLC monitored the reaction process), and after standing to cool to room temperature, the cup-shaped suction filter was used for suction filtration, and suction filtration was performed under 0.1MPa pressure for 20min to obtain 150mg of solid matter; The substance was dissolved in methanol (AR, 2 mL) in a water bath at 50°C, and then recrystallized on standing to obtain 130 mg of the compound having the structure shown in Formula III-1; the yield was 78.8%, and the purity was 95%.
[0121] The structure of formula Ⅱ-1 is The structure of formula III-1 is
[0122] The compound having the structure shown in formula III-1 is a white solid with a melting point of 144-145°C;
[0123] The a...
Embodiment 2
[0129] (1) According to the method of step (1) of Example 1, the compound with the structure shown in formula III-2 is prepared, the only difference is that the compound with the structure shown in formula II-1 is replaced with the compound with the structure shown in formula II-2 compound;
[0130] The structure of formula Ⅱ-2 is The structure of formula III-2 is
[0131] The compound having the structure shown in formula III-2 is a white solid with a melting point of 169-170°C, a yield of 72.3%, and a purity of 95%;
[0132] The analysis results are as follows: 1 H NMR (400MHz, DMSO-d6, ppm) δ9.23(s, 2H, NH, D 2 Oexchangeable), 7.65(d, J=6.6Hz, 2H, Ar-H), 7.46(d, J=8.7Hz, 2H, Ar-H), 4.86(s, 2H, -NH 2 ). 13 C NMR (100MHz, DMSO-d6, ppm) δ179.25, 138.76, 130.71, 125.54, 115.99. HR-MS (ESI), calcd.C 7 h 8 BrN 3 S, [M+Na] + m / z: 267.9520, found: 267.9523.
[0133] (2) According to the method of step (2) of Example 1, the compound having the structure shown in formula...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com