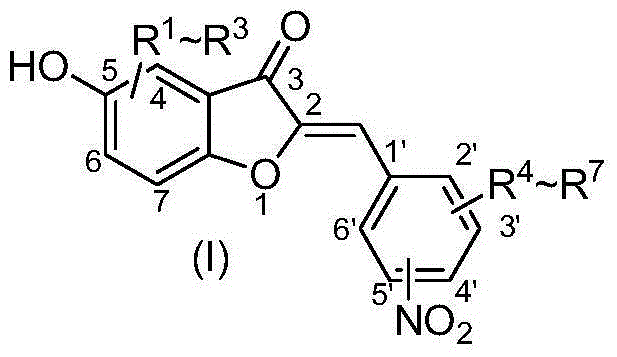
5-hydroxy-2'-nitroaurone or 5-hydroxy-4'-nitroaurone derivatives and application thereof
A nitro and hydroxyl technology, applied in the field of medicinal chemistry, can solve problems such as not having angiogenesis at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: preparation example
[0054]
[0055] Take 1.0 mmol of 5-hydroxyfuranone and 1.2 mmol of the corresponding aldehyde in 10 ml of glacial acetic acid and 4 drops of concentrated hydrochloric acid under the catalysis, stir at room temperature for 3-10 hours, and track the reaction progress by TLC. After the reaction, the reaction mixture was poured into ice water, and the precipitated solid was washed with water and then recrystallized to obtain the target product.
[0056] Compound II-V 1 HNMR data and melting points
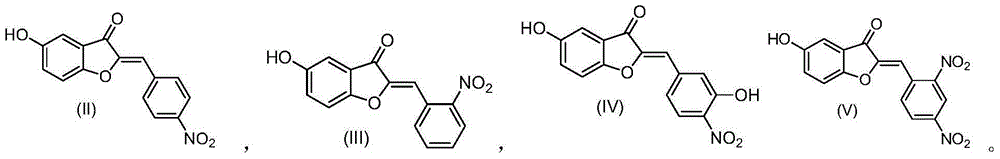
[0057] Compound II
[0058] Melting point: >250°C. 1 HNMR (400MHz, DMSO-d 6 )δ9.90(s,1H),8.35-8.28(m,2H),8.24-8.16(m,2H),7.41(d,J=8.9Hz,1H),7.25(dd,J=8.8,2.7Hz ,1H),7.05(d,J=2.8Hz,1H),7.00(s,1H).
[0059] Compound III
[0060] Melting point: 207.3-208.8°C. 1 HNMR (400MHz, DMSO-d 6 )δ9.89(s,1H),8.23(dd,J=7.8,1.4Hz,1H),8.13(dd,J=8.2,1.3Hz,1H),7.92-7.82(m,1H),7.69(ddd ,J=8.7,7.4,1.4Hz,1H),7.36(d,J=8.8Hz,1H),7.24(dd,J=8.8,2.7Hz,1H),7....
Embodiment 2
[0065] Example 2: Inhibitory activity of compounds II-V on endothelial cells (HUVEC) and tumor cells A549, Bel-74021 and MCF-7
[0066] In a 96-well plate, 1000 HUVEC / well, 1500 MCF-7 or BEL-7402 / well, 2000 A549 / well were inoculated in 190 μL medium, and after culturing for 24 hours, 10 μL blank or different drug concentrations were added. For the test drug, there were 3 groups of parallel blanks for each concentration. After 72 hours of culture, the cells were treated with MTT reagent, and then the OD600 was measured to calculate the IC50.
[0067]
[0068]
Embodiment 3
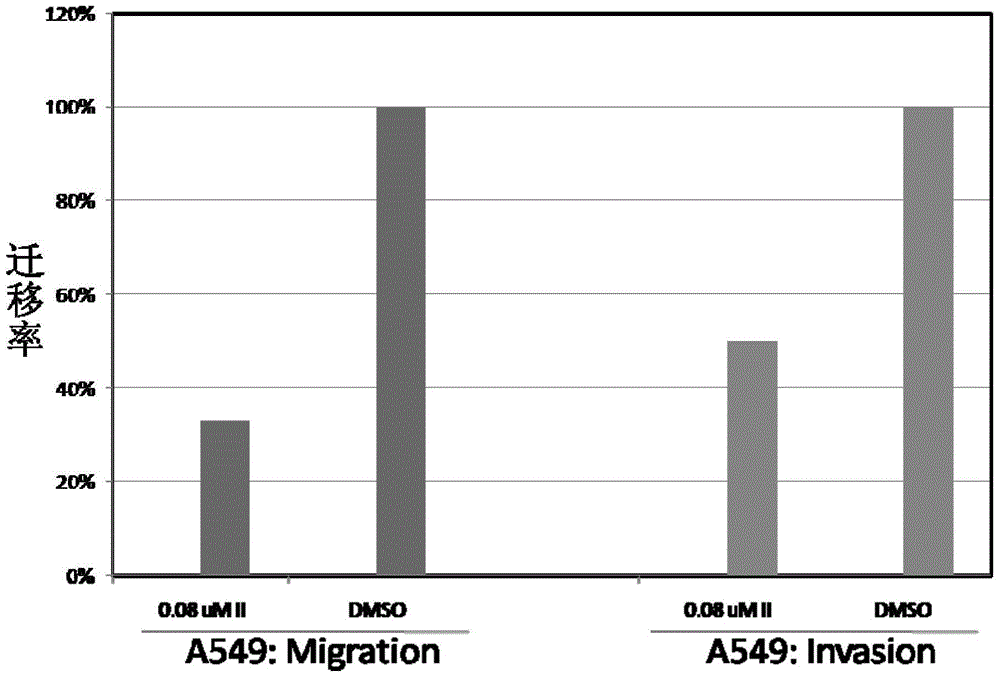
[0070] Ordinary Transwell membrane (8 μmpore) was used for cell migration experiment, and Transwell membrane (8 μmpore) coated with Matrigel (60 μL×250 μg) was used for cell invasion experiment. A549 cells were first starved for 24 hours in a serum-free state, and then 20,000 A549 cells (200 μL) / well were inoculated in Transwell, and the Transwell was placed in a 24-well plate groove containing 600 μL medium (supplemented with 10% FBS) , 0.08 μM compound II was added to A549 cells and cultured for 24 hours. After removing the cells remaining on the upper layer of the Transwell, the Transwell was first fixed with methanol, and then stained with crystal violet. The number of cells in more than 10 regions was counted under a microscope with a magnification of 200 times, and the migration rate was calculated.
[0071] The inhibitory effect of compound II on the migration and invasion of tumor cell A549 is shown in figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com