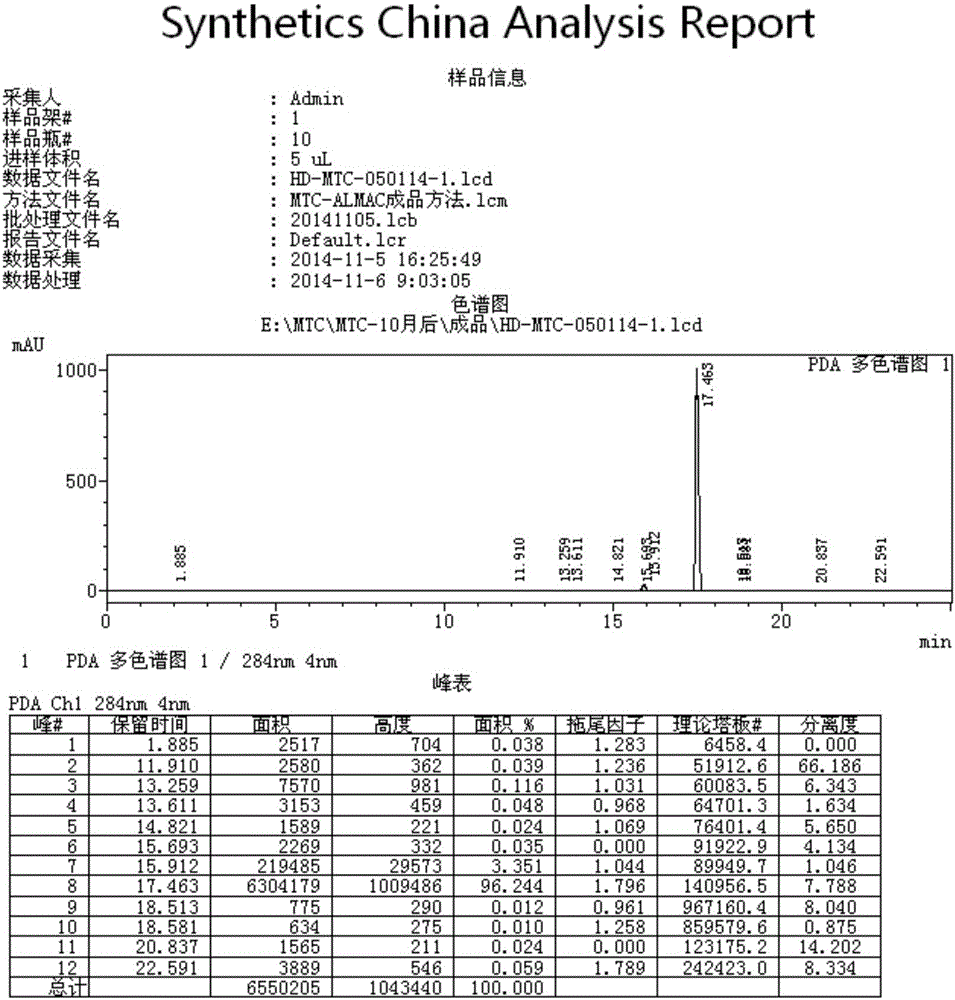
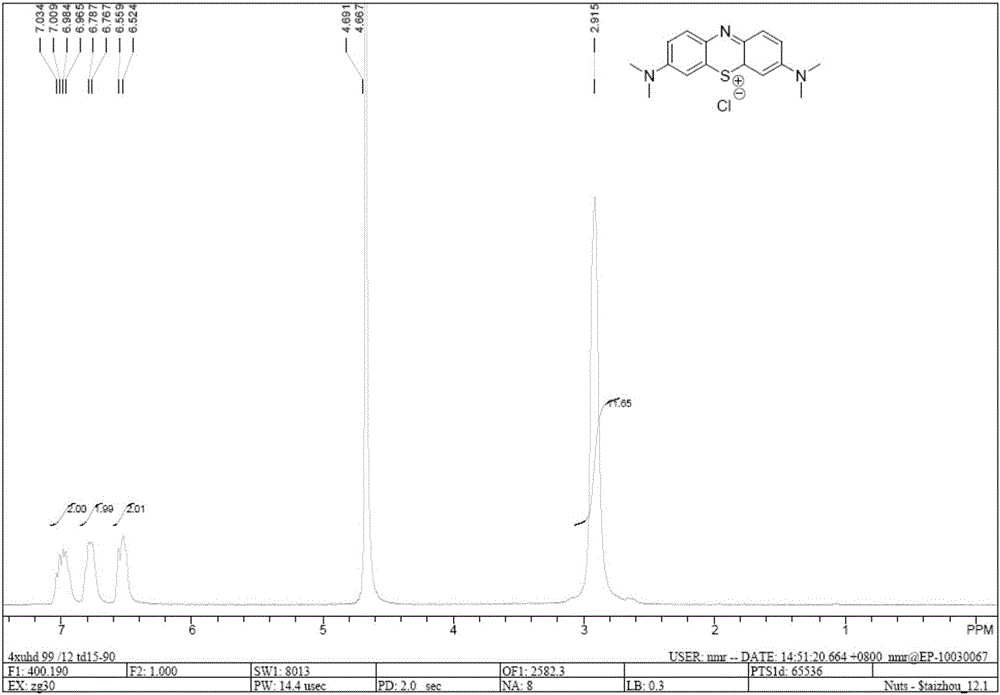
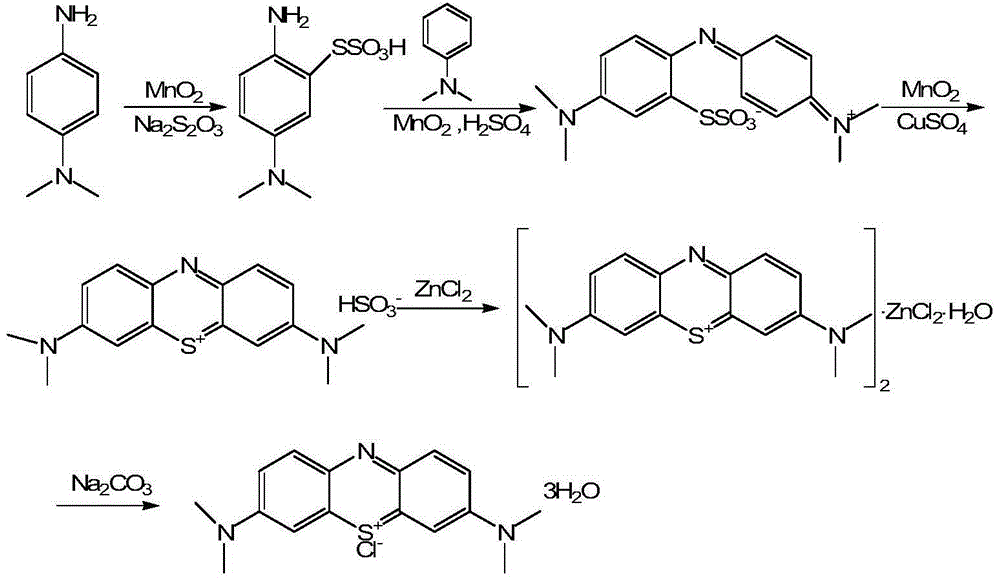
Preparation method of methylene blue
A technology of methylene blue and nitroso, applied in the direction of organic chemistry, can solve the problems of cumbersome feeding process and unfavorable industrial production, and achieve the effect of simple process flow, low environmental pollution and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] 1. Preparation of p-nitroso-N,N-dimethylaniline (3)
[0056] In a 1000mL four-necked bottle, add 116.5g of 36% HCl and 81g of water, stir and cool down to 0-5°C, slowly add 58g of N,N-dimethylaniline dropwise, exothermic during the dropwise addition, the temperature must be strictly controlled for 5 Below ℃, the dropwise addition is completed, stir for 15 minutes, and start to dropwise add a solution of 36.33g sodium nitrite and 113g water. The dropwise exotherm is severe, and the dropping process must be strictly controlled at 0-5°C, and the dropping time is 4-5 hours. , the dropwise addition is completed, and the system temperature is 0-5° C. for 1 h. Filter to remove the mother liquor of hydrochloric acid to obtain solid p-nitroso-N,N-dimethylaniline hydrochloride. In a 1000ml four-necked bottle, add the above-mentioned p-nitroso-N,N-dimethylaniline hydrochloride, 150g toluene and 180g purified water, stir mechanically, start adding 45g aqueous solution of 15g sodiu...
Embodiment 2
[0068] 1. Preparation of p-nitroso-N,N-dimethylaniline (3)
[0069] In a 1000mL four-necked bottle, add 116.5g of 36% HCl and 81g of water, stir and cool down to 0-5°C, slowly add 58g of N,N-dimethylaniline dropwise, exothermic during the dropwise addition, the temperature must be strictly controlled for 5 Below ℃, the dropwise addition is completed, stir for 15 minutes, and start to dropwise add a solution of 36.33g sodium nitrite and 113g water. The dropwise exotherm is severe, and the dropping process must be strictly controlled at 0-5°C, and the dropping time is 4-5 hours. , the dropwise addition is completed, and the system temperature is 0-5° C. for 1 h. Filter to remove the mother liquor of hydrochloric acid to obtain solid p-nitroso-N,N-dimethylaniline hydrochloride. In a 1000ml four-necked bottle, add the above-mentioned p-nitroso-N,N-dimethylaniline hydrochloride, 150g of dichloromethane and 180g of purified water, stir mechanically, start to add 45g of aqueous solu...
Embodiment 3
[0080] 1. Preparation of p-nitroso-N,N-dimethylaniline (3)
[0081] In a 1000mL four-necked bottle, add 116.5g of 36% HCl and 81g of water, stir and cool down to 0-5°C, slowly add 58g of N,N-dimethylaniline dropwise, exothermic during the dropwise addition, the temperature must be strictly controlled for 5 Below ℃, the dropwise addition is completed, stir for 15 minutes, and start to dropwise add a solution of 36.33g sodium nitrite and 113g water. The dropwise exotherm is severe, and the dropping process must be strictly controlled at 0-5°C, and the dropping time is 4-5 hours. , the dropwise addition is completed, and the system temperature is 0-5° C. for 1 h. Filter to remove the mother liquor of hydrochloric acid to obtain solid p-nitroso-N,N-dimethylaniline hydrochloride. In a 1000ml four-necked bottle, add the above-mentioned p-nitroso-N,N-dimethylaniline hydrochloride, 150g ethyl acetate and 180g purified water, stir mechanically, start to add 45g aqueous solution of 15g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com