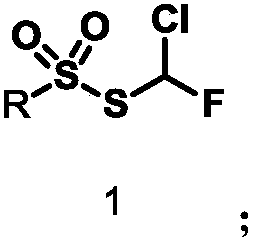
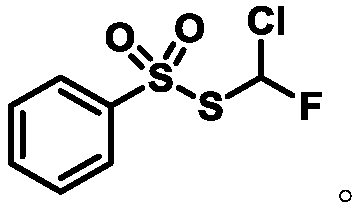
Monochlorofluoromethyl thiosulfonate containing compound, preparation method and application
A compound and alkyl technology, applied in the preparation of sulfides, organic chemistry, etc., can solve problems such as difficulty, little research, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] The preparation of embodiment 1-fluorochloromethylthiobenzenesulfonate
[0122] The preparation of a fluorochloromethylthiobenzenesulfonate is divided into two steps:
[0123] step 1):
[0124]
[0125] Take a 500mL three-necked bottle, add 200mL of n-hexane, and pass through CFCl 2H gas, about 1.5h ventilation. Then take 1 mL of n-hexane solution and add benzotrifluoride as an internal standard. The concentration of the solution is about 3 mmol / mL, and it contains a total of 600 mmol of fluorodichloromethane. Then NaOH (400mmol, 16.0g, 4.0equiv), PhCH 2 SH (100mmol, 12.4g, 1.0equiv), phase transfer catalyst Tris(2-(2-methoxyethoxy)ethyl)amine (8mmol, 2.5g, 0.08equiv), transferred to an oil bath, refluxed at 60°C for 4h. After the reaction, cool to room temperature, spin dry n-hexane, transfer to a 50mL egg-shaped bottle, and distill under reduced pressure with an oil pump (oil bath temperature 70°C, collect fractions at 38-43°C) to obtain 4.0g of a colorless oil...
Embodiment 2
[0131] Example 2 The condition exploration example of reagent and aryl boronic acid coupling reaction
[0132] The general procedure for this coupling reaction is: under the protection of argon, 0.9mmol aryl boronic acid, 0.5mmol reagent, 0.05mmol copper sulfate, 0.75mmol sodium bicarbonate, 5mL methanol in a 25mL sealed tube, react at room temperature for 12h. After the reaction was completed, 10 mL of water was added, extracted with anhydrous ether, dried over anhydrous magnesium sulfate, filtered with diatomaceous earth, concentrated, and the desired product was obtained by column chromatography.
[0133] Examples of conditional exploration are described in Table 1:
[0134] Table 1
[0135]
[0136] In table 1, [M] refers to catalyzer, and R refers to the mol ratio of alkali and reagent, and Base refers to alkali, 19 F yield (%) refers to the yield calculated by tracking the fluorine spectrum.
Embodiment 3
[0138]
[0139] Operation steps: under the protection of argon, weigh 0.9mmol boric acid, 0.5mmol reagent, 0.05mmol copper sulfate, 0.75mmol sodium bicarbonate, 5mL methanol in a 25mL sealed tube, and react at room temperature for 12h. After the reaction, 10 mL of water was added, extracted with anhydrous ether, dried over anhydrous magnesium sulfate, filtered through celite, concentrated, and the residue was subjected to flash silica gel column chromatography to obtain 108 mg of a white solid with a yield of 85%.
[0140] 1 H NMR (400MHz, CDCl 3 )δ7.73-7.57 (m, 5H), 7.50 (t, J = 7.5Hz, 2H), 7.42 (m, 1H), 7.12 (d, J = 56.0Hz, 1H); 19 F NMR (376MHz, CDCl 3 )δ-98.96(d, J=55.9Hz); 13 C NMR (101MHz, CDCl 3 )δ143.03, 139.92, 135.40, 135.39, 129.05, 128.10, 127.84, 127.26, 104.56 (d, J = 282.9Hz) ppm.IR (KBr): v = 1477, 1397, 1227, 1209, 1116, 1000, 837cm -1 .MS(EI):185(100),197,217,252.HRMS(EI):calculated value C 13 h 10 FClS: 252.0176, measured value: 252.0183.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com