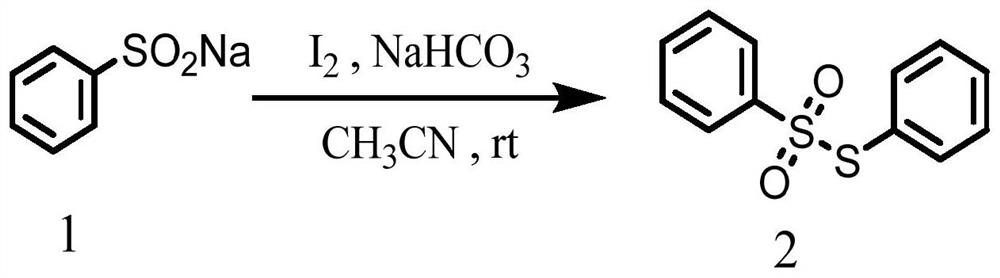
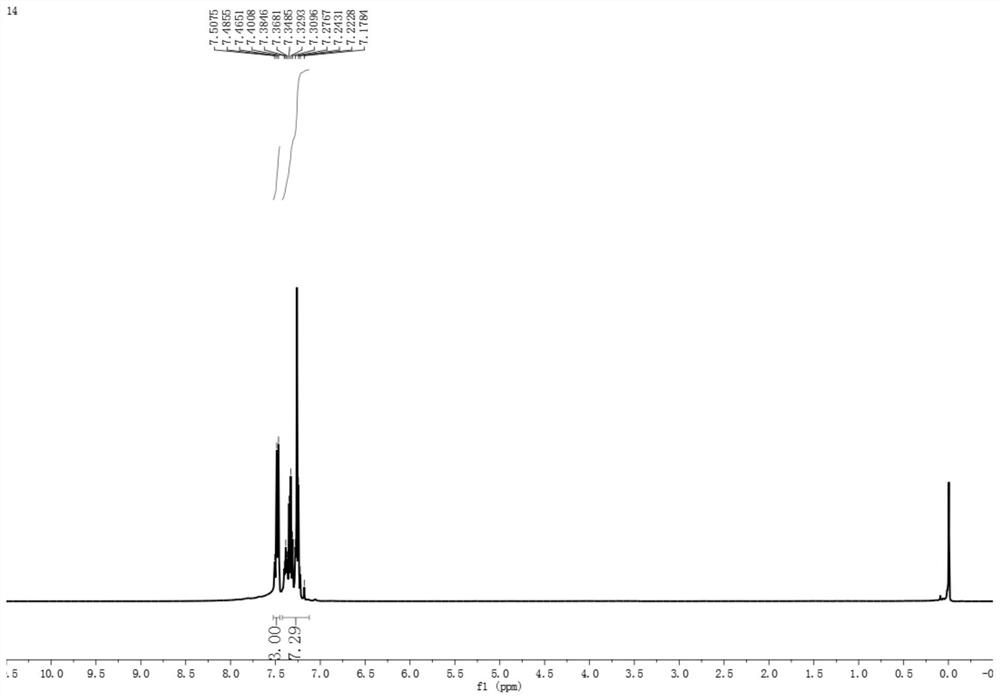
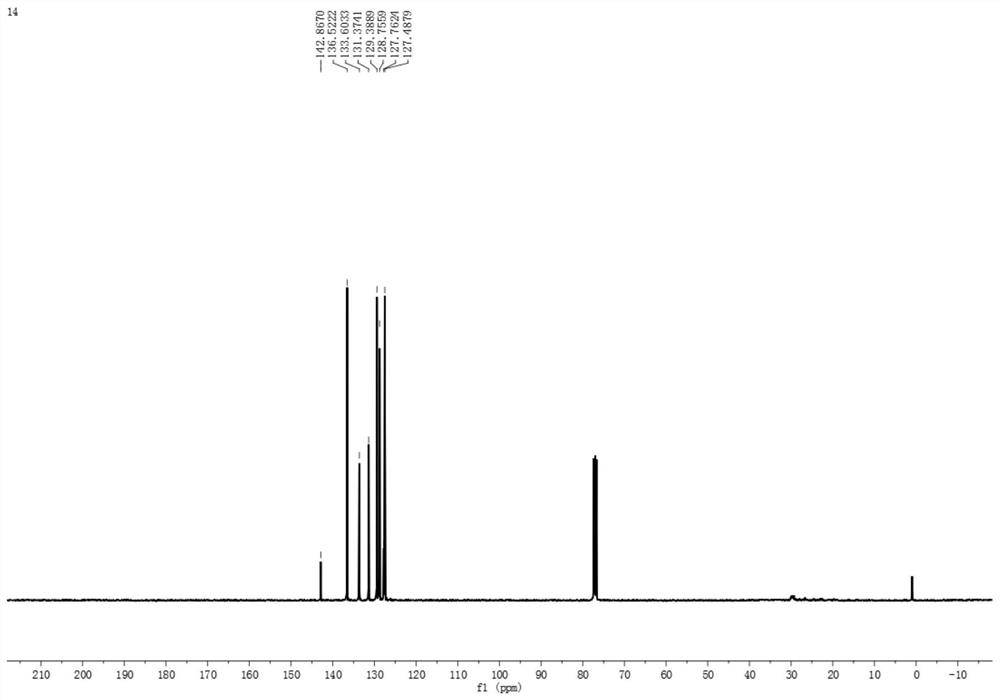
Synthesis method of asymmetric thiosulfonic acid compound
A technology of thiosulfonic acid and synthesis method, applied in the field of synthesis of thiosulfonic acid compounds, can solve the problems of complicated synthesis steps, difficult handling, expensive raw materials and the like, and achieves simplified reaction conditions, easy availability and low price. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A kind of synthetic method of asymmetric thiosulfonic acid compound, comprises the steps:
[0025] (1) 3mL of acetonitrile solvent was added in a 10mL round-bottomed flask, followed by 1mmol of sodium benzene sulfinate;
[0026] (2) add the sodium bicarbonate / iodine of 0.5 equivalent and carry out catalytic reaction according to the equivalent of 1:1, wherein the mol ratio of sodium bicarbonate and iodine is 1:1, the reaction temperature is room temperature (about 22 ℃), and the reaction times is 3.5 h;
[0027] (3) the obtained reactant is cooled to room temperature, and the solvent is evaporated under reduced pressure to obtain the thiosulfonic acid compound crude product;
[0028] (4) Purify the crude product by flash silica gel column chromatography to obtain an asymmetric thiosulfonic acid compound.
[0029] Above-mentioned synthetic reaction scheme such as figure 1 As shown, the reaction conditions were mild without heating or nitrogen blanketing; the yield of ...
Embodiment 2
[0031] A kind of synthetic method of asymmetric thiosulfonic acid compound, comprises the steps:
[0032] (1) in the round bottom flask of 10mL, add 3mL ethanol solvent, then add 1mmol sodium benzene sulfinate;
[0033] (2) add the sodium bicarbonate / iodine of 0.5 equivalent and carry out catalytic reaction according to the equivalent of 1:1, wherein the mol ratio of sodium bicarbonate and iodine is 1:1, the reaction temperature is room temperature (about 22 ℃), and the reaction times is 3.5 h;
[0034] (3) the obtained reactant is cooled to room temperature, and the solvent is evaporated under reduced pressure to obtain the thiosulfonic acid compound crude product;
[0035] (4) Purify the crude product by flash silica gel column chromatography to obtain an asymmetric thiosulfonic acid compound.
[0036] Above-mentioned synthetic reaction scheme such as figure 1 As shown, the reaction conditions were mild without heating or nitrogen blanketing; the yield of product was 26%....
Embodiment 3
[0038] A kind of synthetic method of asymmetric thiosulfonic acid compound, comprises the steps:
[0039] (1) in the round bottom flask of 10mL, add 3mL ethanol solvent, then add 1mmol sodium benzene sulfinate;
[0040] (2) add the sodium bicarbonate / iodine of 0.5 equivalent and carry out catalytic reaction according to the equivalent of 1:1, wherein the mol ratio of sodium bicarbonate and iodine is 1:1, the reaction temperature is room temperature (about 22 ℃), and the reaction times is 3.5 h;
[0041] (3) the obtained reactant is cooled to room temperature, and the solvent is evaporated under reduced pressure to obtain the thiosulfonic acid compound crude product;
[0042] (4) Purify the crude product by flash silica gel column chromatography to obtain an asymmetric thiosulfonic acid compound.
[0043] Above-mentioned synthetic reaction scheme such as figure 1 As shown, the reaction conditions were mild without heating or nitrogen blanketing; the yield of product was 79%....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com