Synthetic method of methyl-2,3,4-trioxy-benzyl-beta-D-pyran riboside
A ribopyranoside and synthetic method technology, which is applied in the field of organic compound synthesis and preparation, can solve problems such as environmental protection, difficulty in feeding materials, and easy to cause danger, achieve high yield, promote reaction progress, and avoid harsh requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The invention provides a method for synthesizing methyl-2,3,4-trioxy-benzyl-β-D-ribopyranoside, comprising: combining 1-methyl-β-D-ribopyranoside and Benzyl chloride reacts under the action of potassium hydroxide and tetrabutylammonium iodide, or under the action of potassium hydroxide and tetrabutylammonium bromide, to prepare methyl-2,3,4-trioxy -Benzyl-β-D-ribopyranoside.
[0021] The present invention uses potassium hydroxide instead of sodium hydride as the base, and simultaneously adds tetrabutylammonium bromide or tetrabutylammonium iodide to successfully prepare methyl-2,3,4-trioxy-benzyl-β-D - Ribopyranoside, which avoids the risk factors caused by the use of sodium hydride and the harsh requirements on environmental humidity, so that the reaction has a higher yield.
[0022] The present invention uses 1-methyl-β-D-ribopyranoside and benzyl chloride as reaction raw materials, and there is no special requirement on the source, which can be commercially availabl...
Embodiment 1
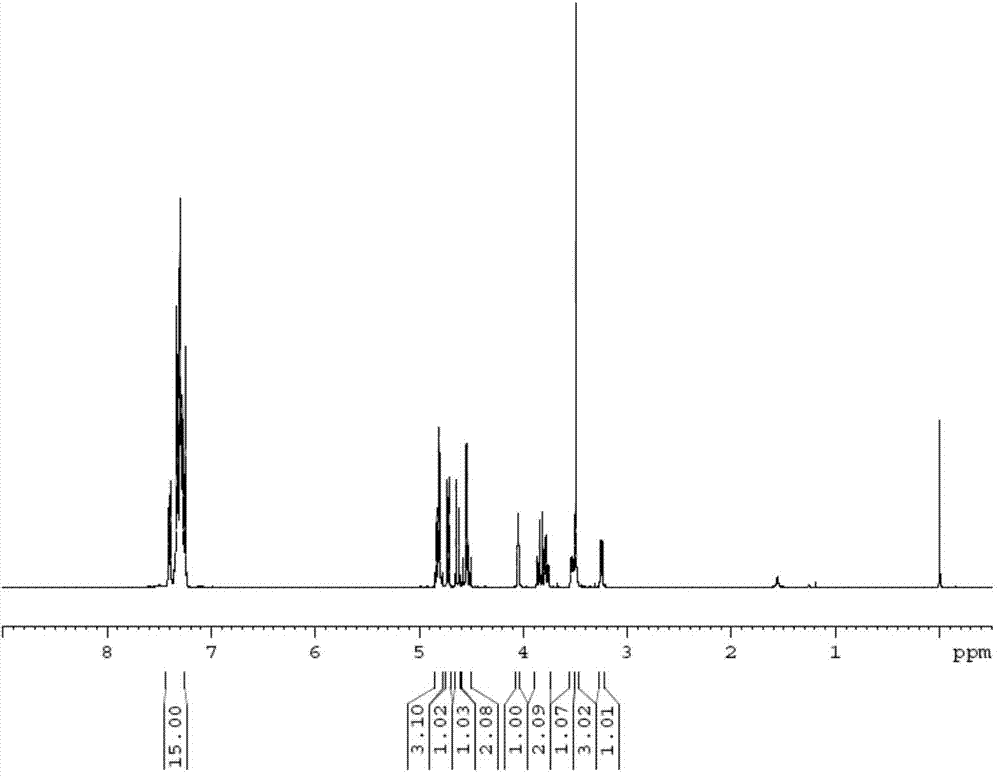
[0036] Add 828g of toluene after dehydration to a 2L three-necked flask with a reflux condenser, and add 80g of 1-methyl-β-D-ribopyranoside under stirring. Cool the system down to room temperature, add 80g of tetrabutylammonium bromide, grind 304g of potassium hydroxide powder to 500-600 mesh, raise the temperature to reflux, then add 203g of benzyl chloride dropwise, and continue the reflux reaction for 1h after the dropwise addition. After the reaction, cool the system down to 40-50°C, wash the toluene layer with 920g*3 deionized water, collect the organic phase and concentrate under reduced pressure to a syrup with a weight loss on drying of less than 10%, and then add 2.5 times the weight of the syrup to the syrup Isohexane, cooled to below 10°C, crystallized and dried to obtain 197.2 g of methyl-2,3,4-trioxo-benzyl-β-D-ribopyranoside with a yield of 93%.
[0037] The structure of the product was characterized by proton nuclear magnetic resonance spectroscopy, the results ...
Embodiment 2
[0039] Add 1500 g of toluene after dehydration to a 2 L three-necked flask with a reflux condenser and a water separator, and add 80 g of 1-methyl-β-D-ribopyranoside under stirring. Cool the system down to room temperature, add 40g of tetrabutylammonium bromide, grind 80g of flake potassium hydroxide to 500-600 mesh, raise the temperature to 50°C, then add 450g of benzyl chloride dropwise, and continue the reflux reaction for 1h after the dropwise addition. After the reaction, the toluene layer was washed with 920g*3 deionized water, the organic phase was collected and concentrated under reduced pressure to a syrup whose lod was less than 10%, and then 2.5 times the weight of the syrup was added to the syrup. crystallized and dried to obtain 205 g of methyl-2,3,4-trioxy-benzyl-β-D-ribopyranoside with a yield of 96.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com