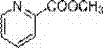
Synthesis method of methyl picolinate
A technology of methyl picolinate and a synthesis method, which is applied in the field of electrochemical organic synthesis, and achieves the effects of cheap and easily available raw materials, reduced air pollution and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 A kind of synthetic method of methyl 2-pyridinecarboxylate, concrete steps are as follows:
[0019] a. Preparation of electrolyte
[0020] Mix 1 mmol (95 ul) 2-bromopyridine with 1 mmol (0.2102 g) tetraethylammonium bromide and 0.129 mol (10 mL) N, N -Dimethylformamide is mixed into an electrolyte, and then placed in a one-chamber electrolytic cell with silver as the cathode and magnesium rod as the anode, 2-bromopyridine, tetraethylammonium bromide and N,N -Dimethylformamide is analytically pure, wherein: 2-bromopyridine is a substrate, N,N -Dimethylformamide is the solvent after 4Å grade molecular sieve drying, and tetraethylammonium bromide is the supporting electrolyte;
[0021] b. Electrocarboxylation reaction
[0022] Under normal pressure, carbon dioxide was introduced into the above electrolytic cell for 30 min, and then at 8 mA / cm 2 Electrolyze at a constant current density of 2.0 F per mole of 2-bromopyridine, and F is the Faraday constant; ...
Embodiment 2
[0029] Embodiment 2 A kind of synthetic method of methyl 2-pyridinecarboxylate, concrete steps are as follows:
[0030] a. Preparation of electrolyte
[0031] Mix 1 mmol (95 ul) 2-bromopyridine with 1 mmol (0.3224 g) tetrabutylammonium bromide and 0.129 mol (10 mL) N,N -Dimethylformamide is mixed into an electrolyte, and then placed in a one-chamber electrolytic cell with a silver sheet as a cathode and a magnesium rod as an anode, 2-bromopyridine, tetrabutylammonium bromide and N,N -Dimethylformamide is analytically pure, wherein: 2-bromopyridine is a substrate, N,N -Dimethylformamide is the solvent after 4Å grade molecular sieve drying, and tetrabutylammonium bromide is the supporting electrolyte;
[0032] b. Electrocarboxylation reaction
[0033] Under normal pressure, carbon dioxide was introduced into the above electrolytic cell for 30 min, and then at 8 mA / cm 2 Electrolyze at a constant current density of 2.0 F per mole of 2-bromopyridine, and F is the Faraday consta...
Embodiment 3
[0040] Embodiment 3 A kind of synthetic method of methyl 2-pyridinecarboxylate, concrete steps are as follows:
[0041] Mix 1 mmol (95 ul) 2-bromopyridine with 1 mmol (0.3224 g) tetrabutylammonium bromide and 0.129 mol (10 mL) N,N -Dimethylformamide is mixed into an electrolyte, and then placed in a one-chamber electrolytic cell with nickel as the cathode and magnesium rod as the anode, 2-bromopyridine, tetrabutylammonium bromide and N,N -Dimethylformamide is analytically pure, wherein: 2-bromopyridine is a substrate, N,N -Dimethylformamide is the solvent after 4Å grade molecular sieve drying, and tetrabutylammonium bromide is the supporting electrolyte;
[0042] b. Electrocarboxylation reaction
[0043] Under normal pressure, carbon dioxide was introduced into the above electrolytic cell for 30 min, and then at 8 mA / cm 2 Electrolyze at a constant current density of 2.0 F per mole of 2-bromopyridine, and F is the Faraday constant;
[0044] c. Esterification reaction
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com