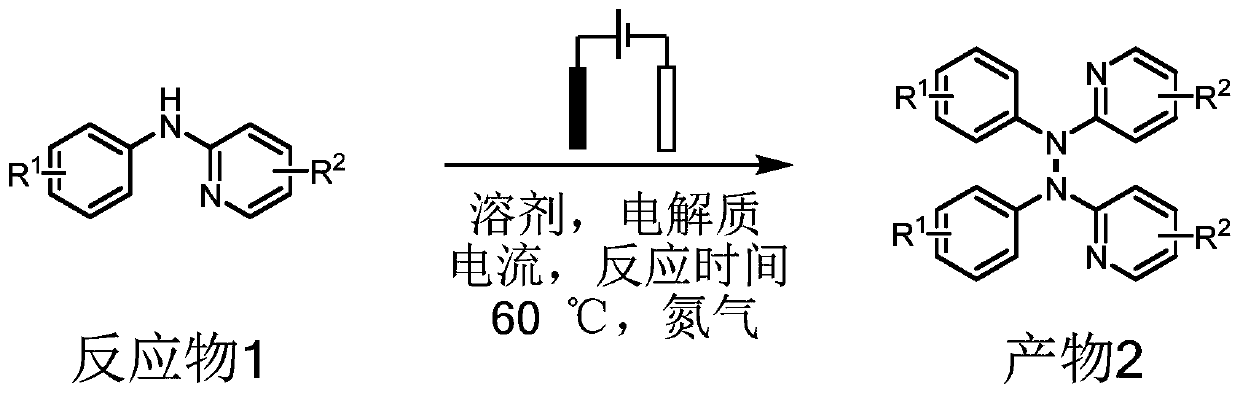
Method for electrochemical synthesis of tetraarylhydrazine compound
A compound and aryl hydrazine technology, applied in the field of electrochemical organic synthesis, can solve the problems of expensive catalyst, unfriendly environment, and small application range of substrates, and achieve a simple and efficient reaction system, easy availability of raw materials, and wide application scope of substrates. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Add 0.25mmol of N-(4-chlorophenyl)-5-fluoropyridin-2-amine, 0.5mmol of tetrabutylammonium iodide as an electrolyte, 7.0mL of acetonitrile, and 0.5mL of methanol in a three-necked flask And a magnetic stirrer, nitrogen as a protective gas, a platinum sheet (1.0cm×1.0cm) as an anode and a cathode, turn on the power, adjust the current to 5.0mA, and react at 60°C for 10 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the crude product was purified by column chromatography. The eluent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 10:1 to obtain a tetraarylhydrazine compound with a yield of 99%. 2a.
[0061]
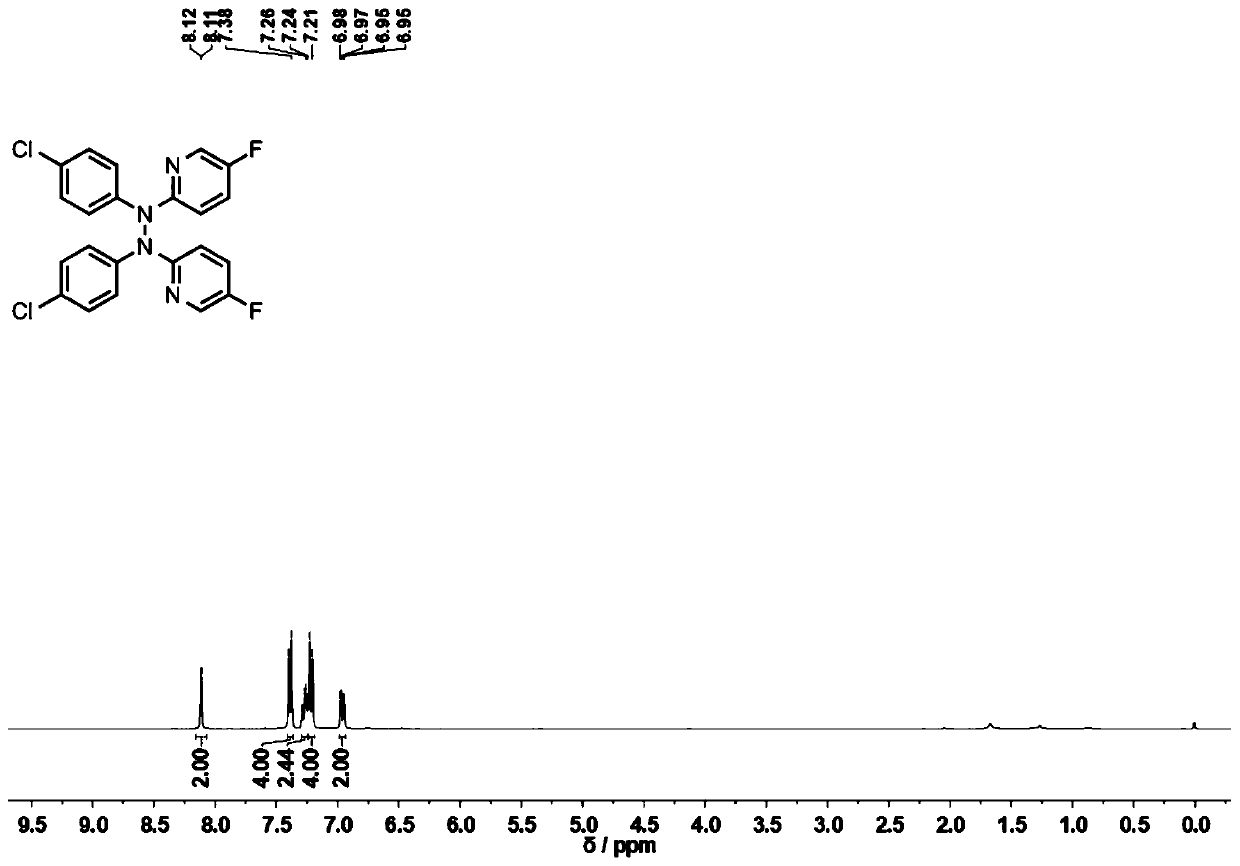
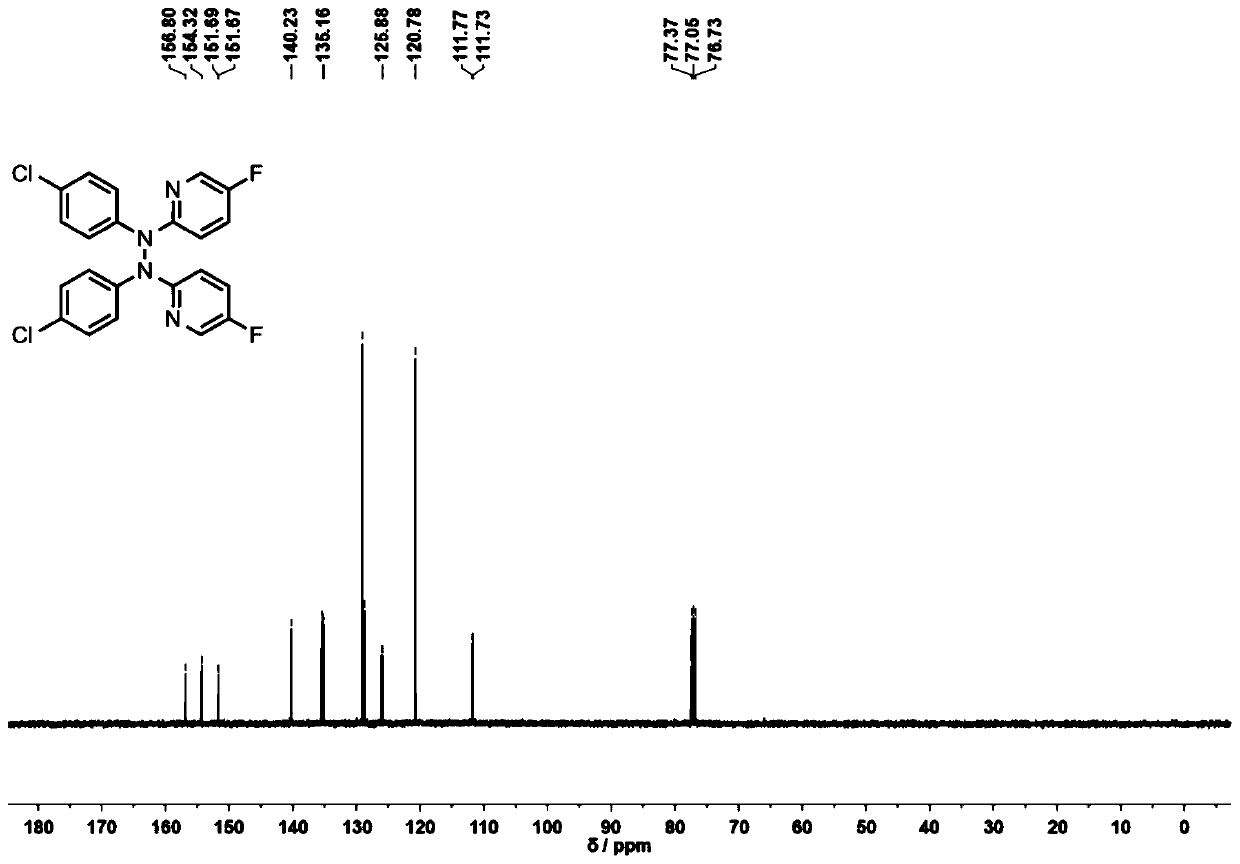
[0062] The product 2a that present embodiment obtains 1 H-NMR spectrum such as figure 2 as shown, 13 C-NMR spectrum such as image 3 As shown, the NMR data are as follows:
[0063] 1 H-NMR (400MHz, CDCl 3 ): δ8.11(d, J=2.6Hz, 2H), 7.39(d, J=8.7Hz, 4H), 7.30-7.24(m, 2H), 7.22(d, J=8...
Embodiment 2
[0067] Into a three-necked flask were sequentially added 0.25 mmol of 5-fluoro-N-(p-tolyl)pyridin-2-amine, 0.5 mmol of tetrabutylammonium iodide as an electrolyte, 7.0 mL of acetonitrile, 0.5 mL of methanol, and a magnetic Stirrer, nitrogen as protective gas, platinum sheet (1.0cm×1.0cm) as anode and cathode, power on, adjust current to 5.0mA, react at 60°C for 10 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the crude product was purified by column chromatography. The eluent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 10:1 to obtain 92% tetraarylhydrazine compound 2b, structural formula as follows:
[0068]
[0069] Product 2b 1 H-NMR spectrum such as Figure 4 as shown, 13 C-NMR spectrum such as Figure 5 As shown, the NMR data are as follows:
[0070] 1 H-NMR (400MHz, CDCl 3 ):δ8.09(d,J=2.8Hz,2H),7.35(d,J=8.4Hz,4H),7.25-7.19(m,2H),7.07(d,J=8.3Hz,4H),6.95 (dd,J=9.1,3.4Hz,2H),2.27(s,6...
Embodiment 3
[0074] 0.25mmol of N-(4-(tert-butyl)phenyl)-5-fluoropyridin-2-amine, 0.5mmol of tetrabutylammonium iodide as electrolyte, 7.0mL of acetonitrile, 0.5mL of methanol and a magnetic stirrer, nitrogen as a protective gas, platinum sheets (1.0cm×1.0cm) as anode and cathode, turn on the power, adjust the current to 5.0mA, and react at 60°C for 10 hours. After the reaction was completed, the solvent was removed by rotary evaporation, and the crude product was purified by column chromatography. The eluent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 10:1 to obtain a tetraarylhydrazine compound with a yield of 95%. 2c, the structural formula is as follows:
[0075]
[0076] Product 2c 1 H-NMR spectrum such as Image 6 as shown, 13 C-NMR spectrum such as Figure 7 As shown, the NMR data are as follows:
[0077] 1 H-NMR (400MHz, CDCl 3 ): δ8.11(d, J=2.6Hz, 2H), 7.42(d, J=8.6Hz, 4H), 7.28(d, J=8.7Hz, 4H), 7.24-7.18(m, 2H), 6.96 (dd,J=9.1,3.3Hz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com