Preparation method of 1-bromoalkyne and 1-iodoalkyne
A technology for substituting alkynes and alkynes, applied in the field of organic synthetic chemistry, can solve the problems of toxic and harmful halogenated reagents, harsh reaction conditions, complex experimental operations, etc., and achieve the effects of environmental friendliness, simple operation, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Synthesis of 1-chloro-2-(iodoethynyl)benzene (1a)
[0031]
[0032] Add 1-chloro-2-ethynylbenzene (68mg, 0.5mmol), acetonitrile (3mL), chloramine B (160mg, 0.75mmol) and potassium iodide (99mg, 0.6mmol) into a 25mL Schlenk bottle successively, and then next reaction,
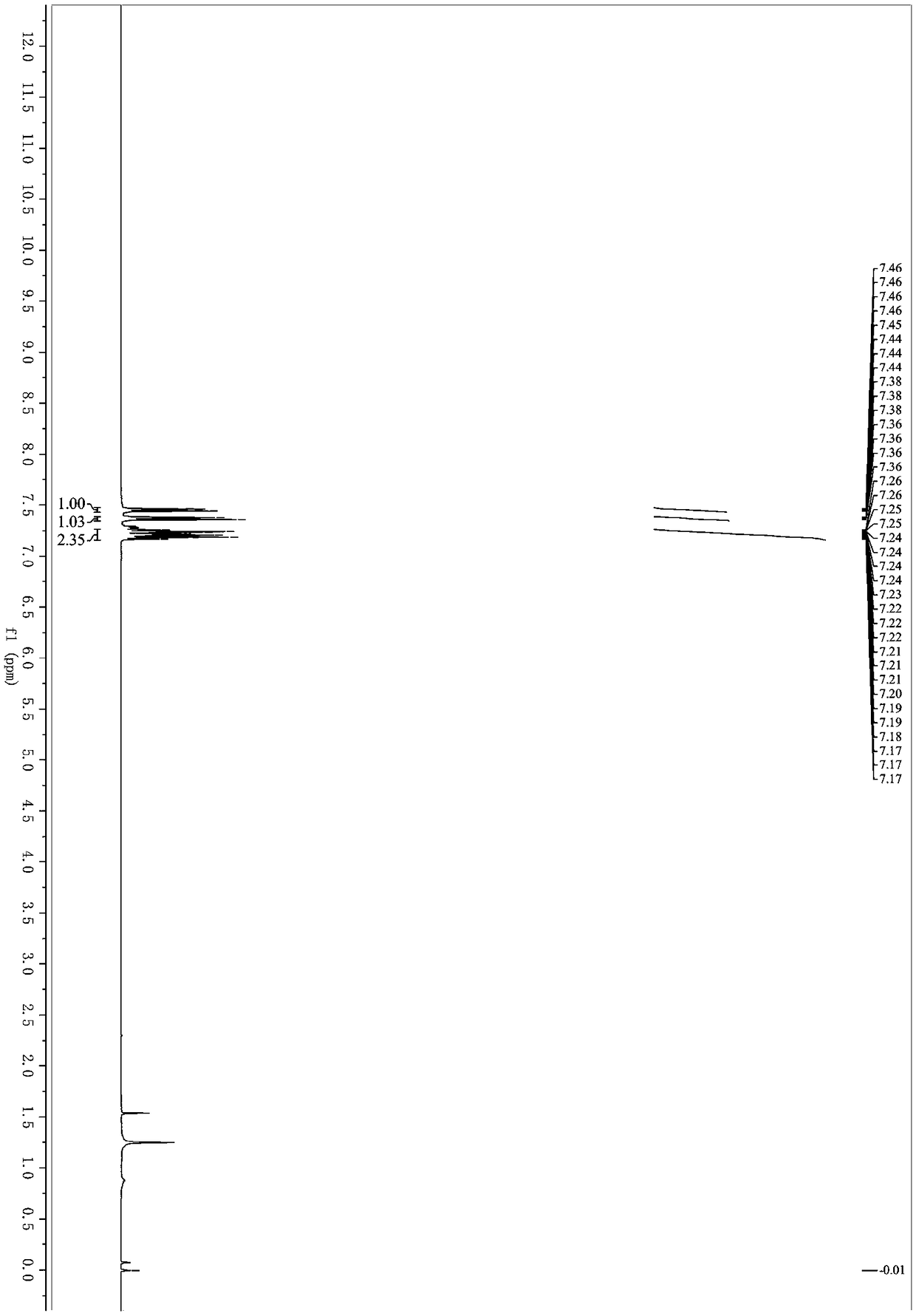
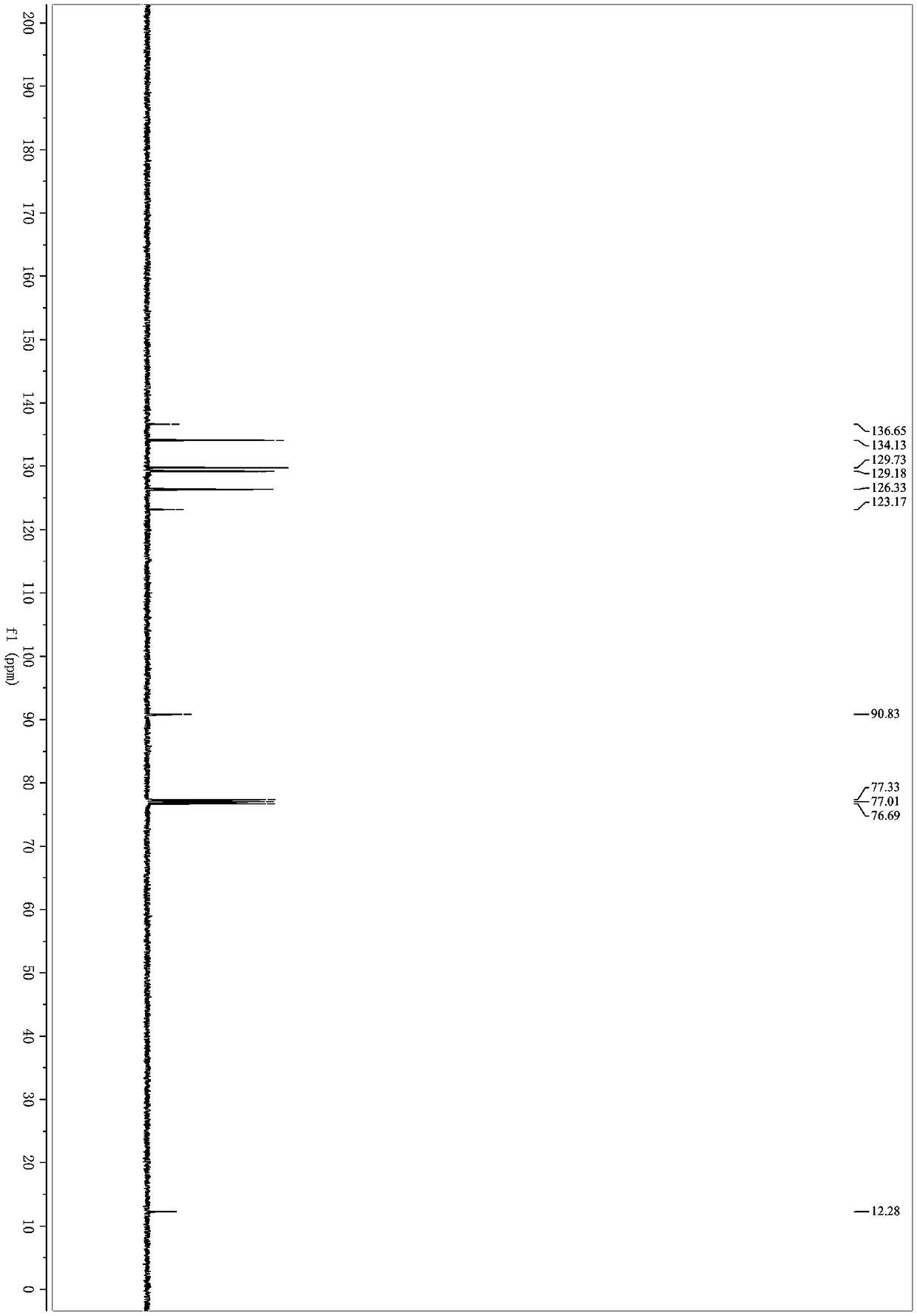
[0033] React for 2 hours. After the reaction, filter and remove the organic solvent under reduced pressure; elute with petroleum ether / ethyl acetate and separate on silica gel column. The yield of 1-chloro-2-(iodoethynyl)benzene is 95%. 1 H NMR (400MHz, Chloroform-d) δ7.45 (ddd, J=7.1, 2.1, 0.9Hz, 1H), 7.37 (dt, J=7.8, 1.1Hz, 1H), 7.27–7.14 (m, 2H). 13 C NMR (101MHz, cdcl 3 )δ136.65, 134.13, 129.73, 129.18, 126.33, 123.17, 90.83, 12.28. HRMS-ESI (m / z): calcd for C 8 h 5 ClI[M+H] + :262.9119, found 262.9120.
Embodiment 2
[0034] Example 2: Synthesis of 1-(iodoethynyl)-3-toluene (1b)
[0035]
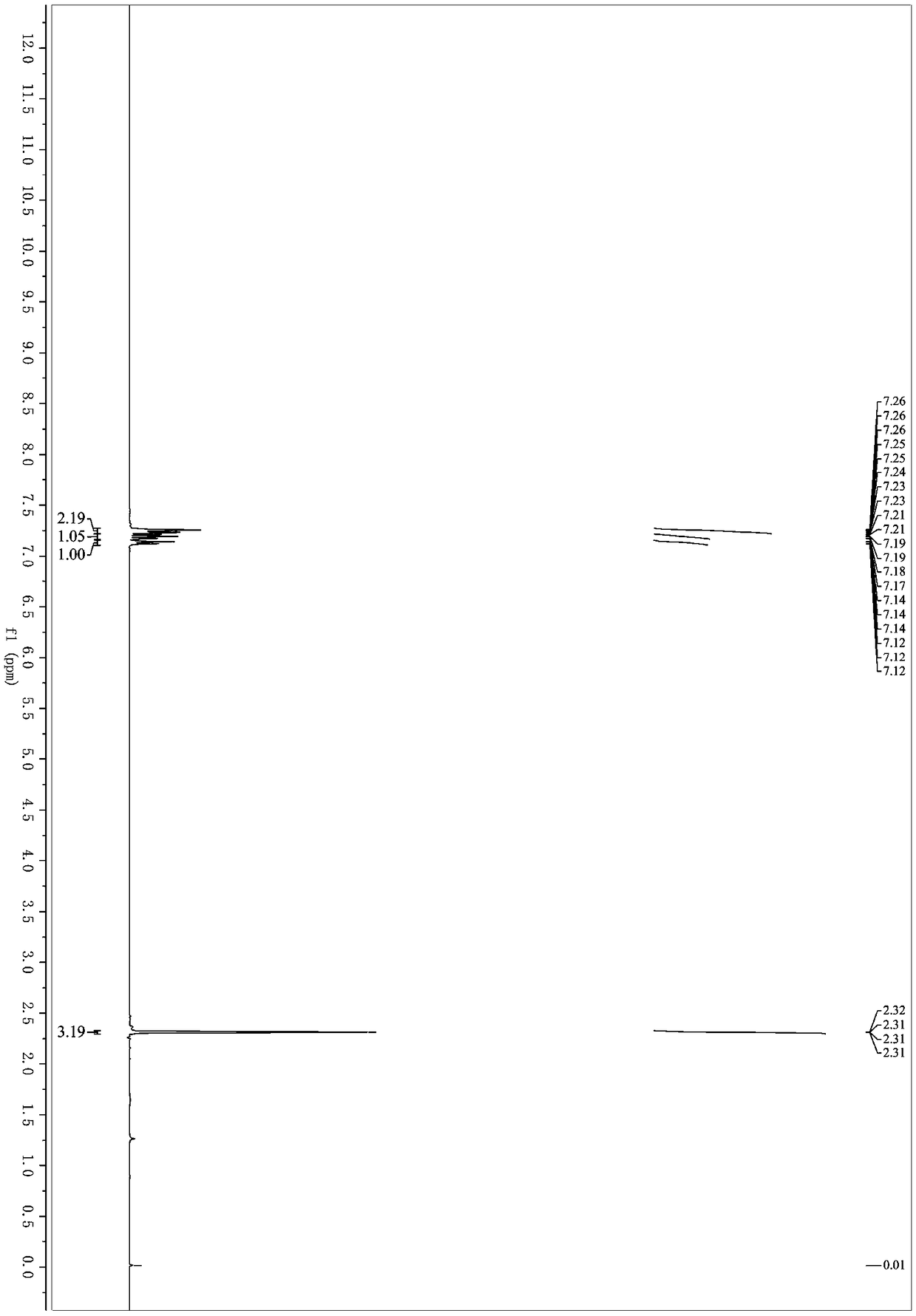
[0036] Add 1-acetylene-3-toluene (58mg, 0.5mmol), acetonitrile (3mL), chloramine B (160mg, 0.75mmol) and potassium iodide (99mg, 0.6mmol) into a 25mL Schlenk bottle successively, and react at room temperature , reacted for 2 hours, after the reaction was over, filtered, and the organic solvent was removed under reduced pressure; eluting with petroleum ether / ethyl acetate, separated by silica gel column, the yield of 1-(iodoethynyl)-3-toluene was 98%. 1 H NMR (400MHz, Chloroform-d) δ7.27–7.22 (m, 2H), 7.19 (td, J=7.5, 1.3Hz, 1H), 7.16–7.11 (m, 1H), 2.31 (s, 3H). 13 C NMR (101MHz, cdcl 3 )δ137.94, 132.89, 129.72, 129.38, 128.13, 123.17, 94.34, 21.22, 5.69.HRMS-ESI(m / z): calcd for C 9 h 8 I[M+H] + :242.9665, found 242.9662.
Embodiment 3
[0037] Example 3: Synthesis of 3-(iodoethynyl)thiophene (1c)
[0038]
[0039]3-Ethynylthiophene (54mg, 0.5mmol), acetonitrile (3mL), chloramine B (160mg, 0.75mmol) and potassium iodide (99mg, 0.6mmol) were successively added to a 25mL Schlenk bottle, and then reacted at room temperature. After 2 hours, after the reaction was completed, filter and remove the organic solvent under reduced pressure; elute with petroleum ether / ethyl acetate, separate on a silica gel column, and the yield of 3-(iodoethynyl)thiophene is 95%. 1 H NMR (400MHz, Chloroform-d) δ7.46 (dd, J = 2.9, 1.4Hz, 1H), 7.24 (dd, J = 5.2, 1.4Hz, 1H), 7.10 (dt, J = 5.1, 1.3Hz, 1H). 13 C NMR (101MHz, cdcl 3 )δ130.30, 129.98, 125.16, 122.49, 89.15, 5.81. HRMS-ESI (m / z): calcd for C 6 h 4 IS[M+H] + :234.9073,found234.9073.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com