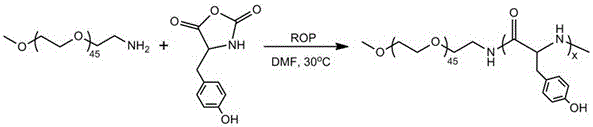
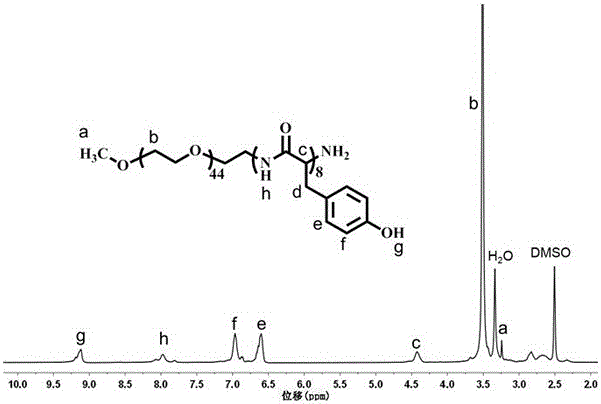
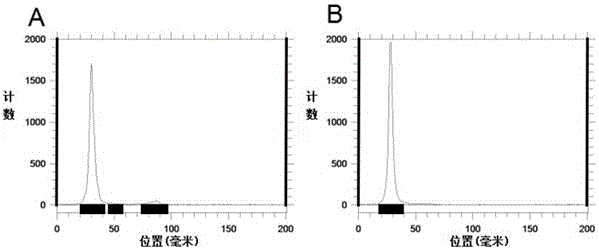
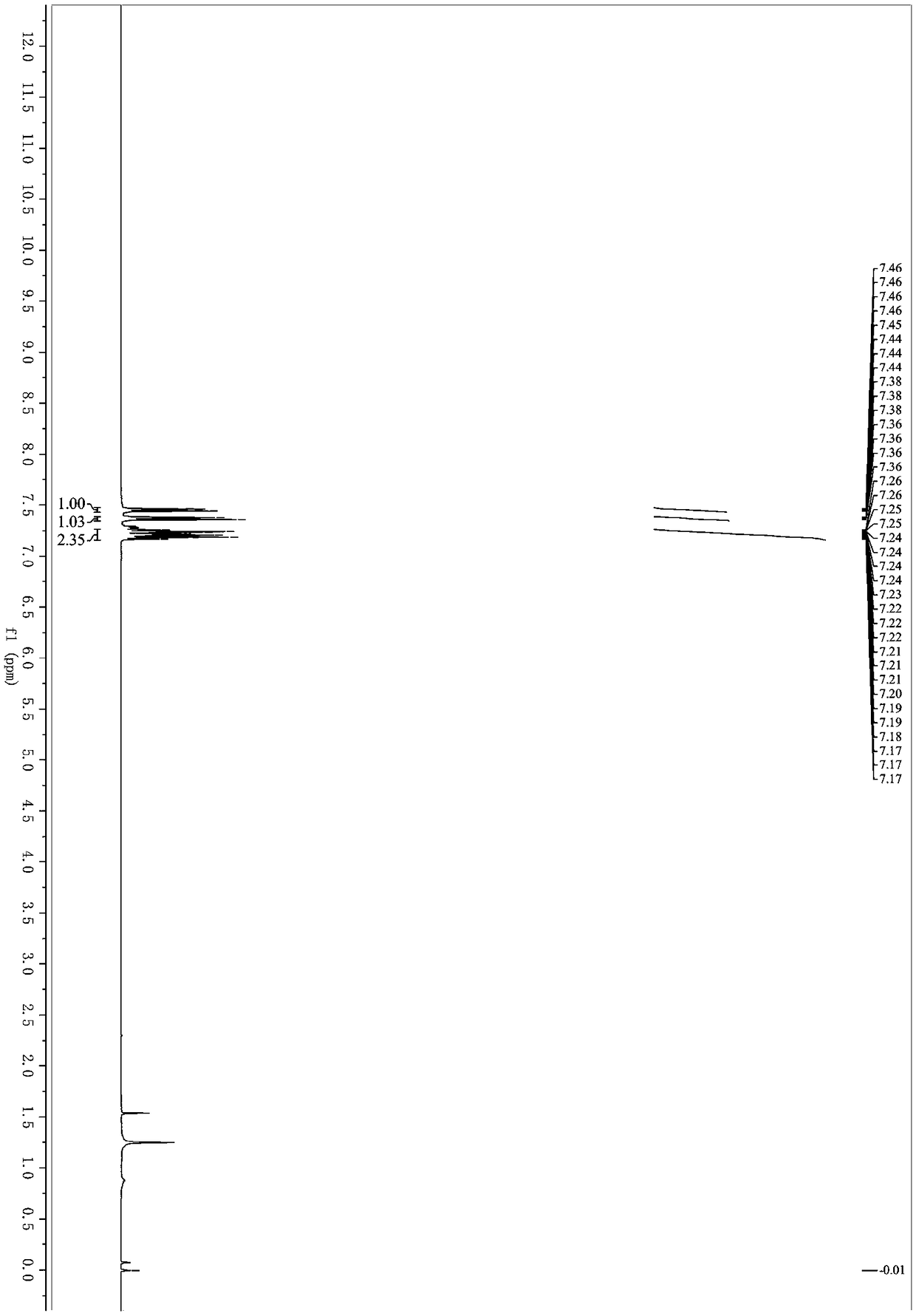
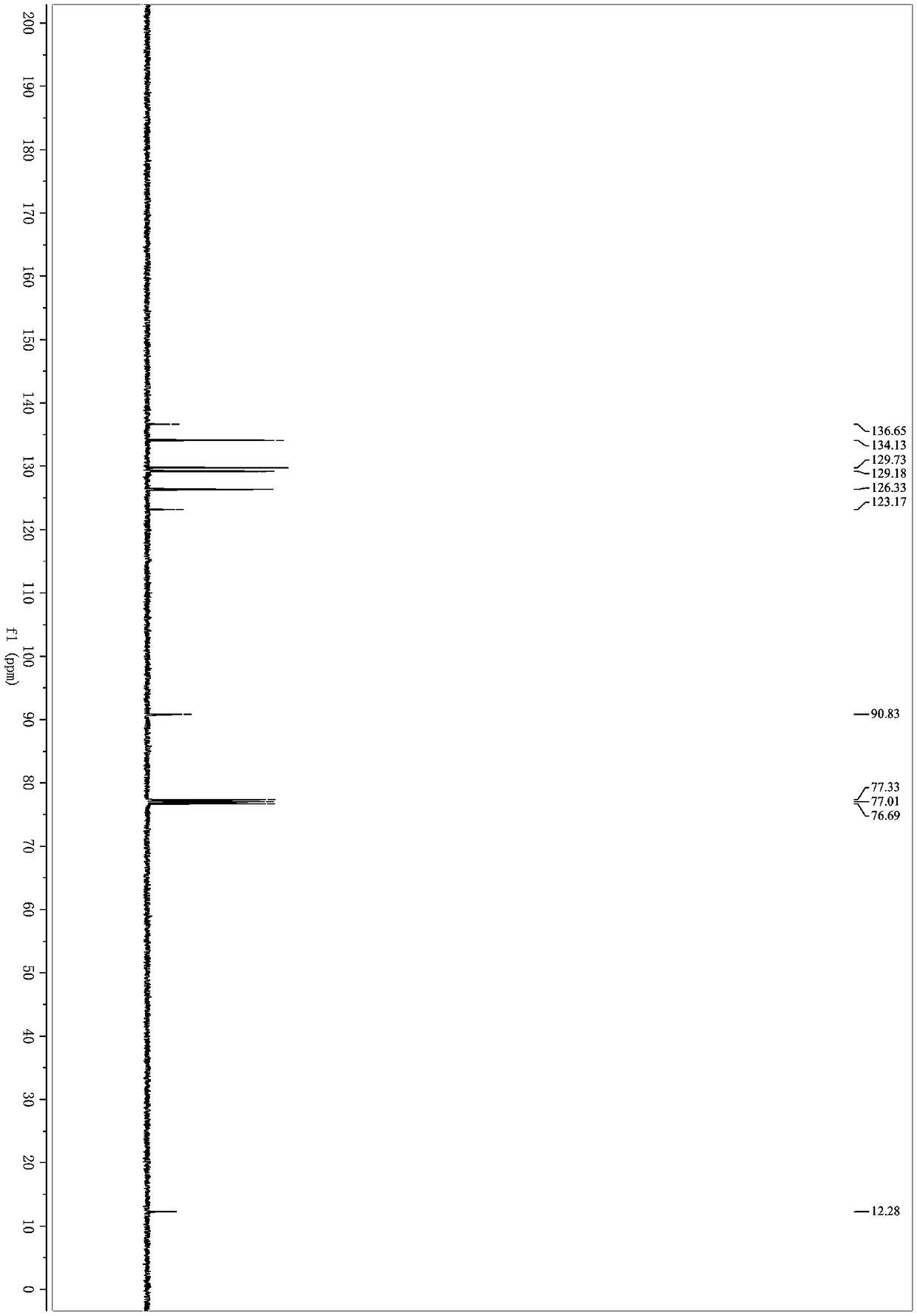
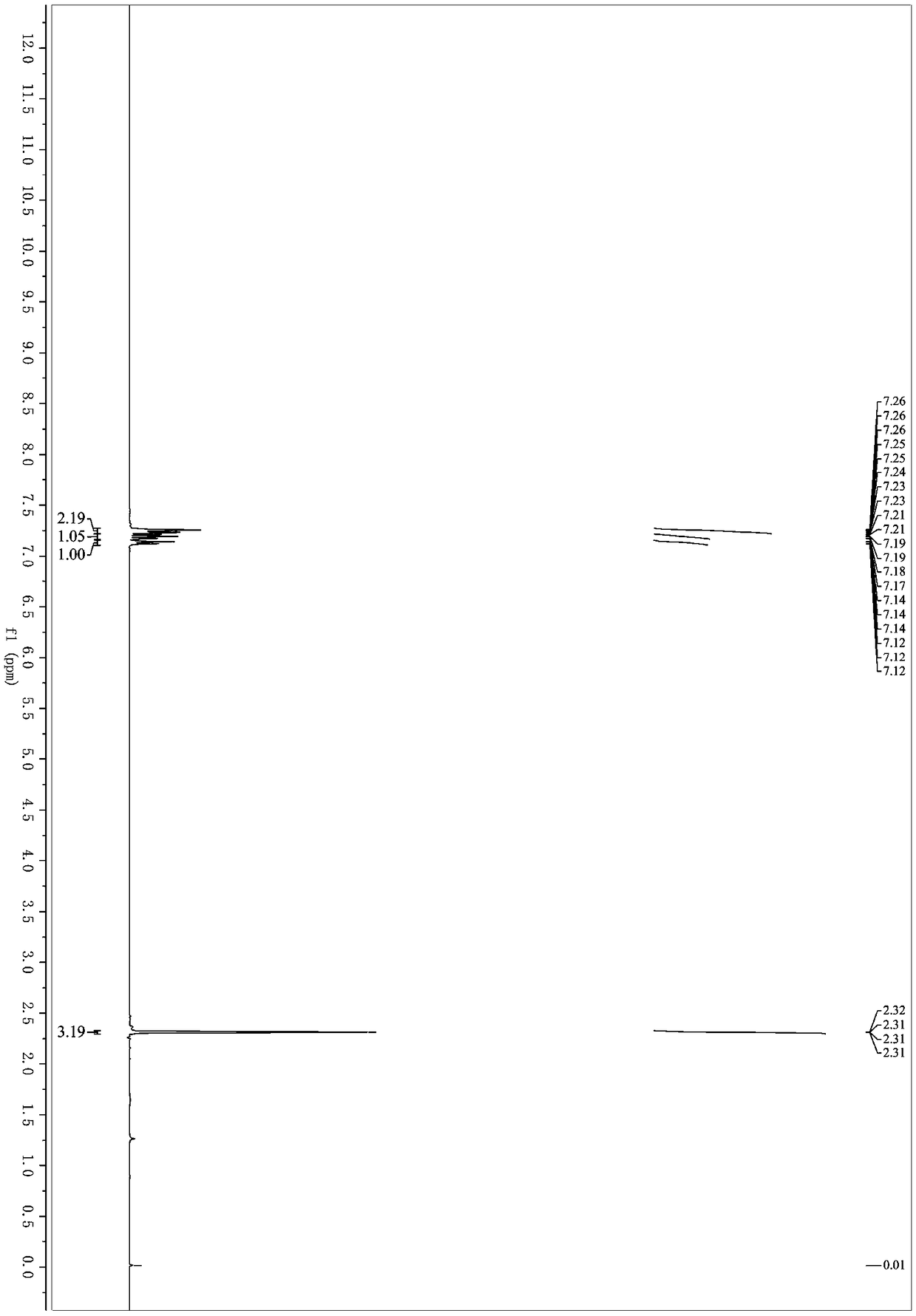
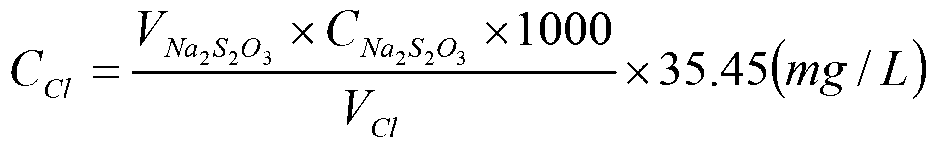Patents
Literature
64 results about "Chloramine-T" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
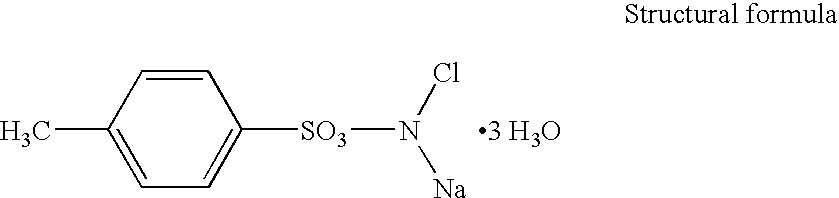
Chloramine-T is the organic compound with the formula CH₃C₆H₄SO₂NClNa. Both the anhydrous salt and its trihydrate are known. Both are white powders. Chloramine-T is used as a reagent in organic synthesis.
Culture solution and preservation solution for coccidian oocyst
The invention discloses a coccidian ovisac culture liquid and conservative liquid, which comprises the following steps: adapting this culture liquid and conservative liquid at (0.5-8.0):100 with chloramine T and pure water to form chloramine T solution; separating and purifying fresh coccidian oocyst from ovisac fowl dung; suspending the ovisac sediment in the chloramine T solution at 25-30 deg.c; setting the culture wetness more than 50 percent and ovisac density less than 1000,000 oocyst per ml and culture liquid depth less than 0.7 cm; stirring culture liquid more than three times a day for 24-72 h; stopping cultivating when more than 80 percent oocyst finishes sporing; obtaining oocyst culture liquid; centrifuging condensation; adding chloramine T solution again; stewing at 4-8 deg.c in the fridge. The invention improves the sporing rate of coccidian ovisac culture liquid, which avoids heavy metal material in the chitchen and environmental pollution.
Owner:FOSHAN STANDARD BIO TECH
Chloramine T based teat cleaner and related process
ActiveUS20040225017A1Easy compoundSubstantial saving in shipping costBiocideAmine active ingredientsBacteroidesMastitis
Dairy animals have been milked since prehistory. Because these dairy animals are often kept in confined areas the teat areas of these animals are often contaminated with bacteria. This invention is concerned with a process for sanitizing these contaminated teat areas by treating these areas with a solution of Chloramine T. The preferred solutions for sanitizing the teat areas have concentrations of from about 0.005 to about 1 weight percent. Treating solutions for use in this invention may further incorporate a coloring agents, wetting agent, surfactants, healing agents, dyes, thickening agents, skin conditioning agents, softeners etc. The process of this invention is fast acting and is effective against a wide spectrum of bacteria. After treatment the teat area of the dairy animal being milked is bacteria free and hence bacterial contamination of the milk, and Mastitis infections are eliminated.
Owner:IOFINA CHEM
Detection reagent for fast detecting cyanide in water and preparation method of detection reagent
InactiveCN102621137AWide detection rangeExtended shelf lifeMaterial analysis by observing effect on chemical indicatorCelluloseChloramine
The invention relates to a detection reagent for fast detecting cyanide in water. The detection reagent comprises a release reagent and a color developing reagent, wherein the release reagent comprises monopotassium phosphate, methylcellulose, lactic acid and acetone mixed solvents and chloramine T, the color developing reagent comprises sodium hydroxide, isonicotinic acid, barbituric acid and methylcellulose, and the release reagent and the color developing reagent are encapsulated in a separated way according to the consumption for each time. The release reagent comprises the preparation steps that: 1, major ingredients and deionized water are mixed and ground into paste; 2, the paste is placed in a vacuum drying box for drying; 3, dried materials are cooled and ground into powder; 4, the lactic acid and acetone mixed solvents are used for preparing 5% chloramine T solution; and 5, the chloramine T solution is added into the prepared powder, the materials are dried in the air, and the powdery release reagent is obtained. The preparation steps of the release reagent refer to the partial steps. The detection reagent disclosed by the invention can be matched with a colorimetric tube and a standard colorimetric card to be used, the carrying is convenient, the detection range is wide, the time is short, and convenience can be provided for the fast detection of the cyanide in water in various sites.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation of 131I-thyroid stimulating hormone (TSH) and application thereof
InactiveCN101787077ASimple preparation processStable markerRadioactive preparation carriersDepsipeptidesTyrosineUndifferentiated Thyroid Tumor
The invention relates to the preparation of 131I-thyroid stimulating hormone (TSH) and an application thereof, belonging to the field of radionuclide therapeutic drugs. The compound 131I-thyroid stimulating hormone is a radioiodine marker for thyroid stimulating hormone with pathoclisis in vivo acting on thyrocyte. A preparation method of the 131I-thyroid stimulating hormone comprises the following steps of: carrying out iodine 131 marking on the thyroid stimulating hormone by utilizing a chloramine-T method, a peroxide oxidation method or an iodogen method, and introducing radionuclide iodine 131 for treatment into tyrosine residues in thyroid stimulating hormone molecules. The marking rate of the prepared 131I-TSH is 85.9 percent, the radiochemical purity after purification achieves more than 90 percent, and the prepared 131I-TSH can be stored stably for more than one week at room temperature; the 131I-TSH is mainly metabolized through the liver and excreted through the kidneys; the percentage of ID / g of the 131I-TSH in the thyroid gland is not obviously reduced in four hours; and the 131I-TSH can be concentrated in thyroid gland issues sealed by an iodine solution. Concerning low / undifferentiated thyroid tumours incapable of iodine uptake, the 131I-TSH can increase the radioiodine uptake of thyroid gland cancer cells, and the radioiodine 131 can be chosen for treatment.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
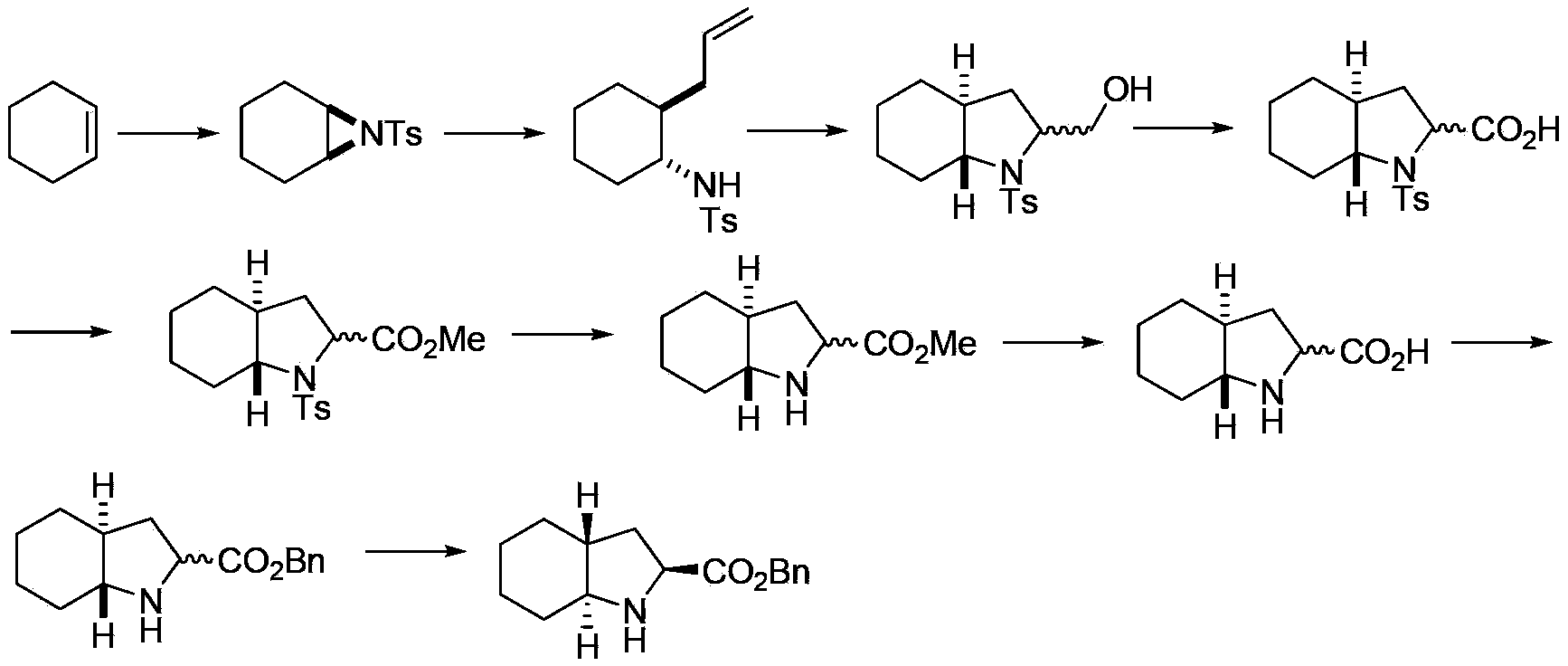
Method for preparing trandolapril intermediate
ActiveCN102887853AHigh yieldHigh stereoselectivityOrganic chemistryBulk chemical productionCyclohexylaminesAcyl group
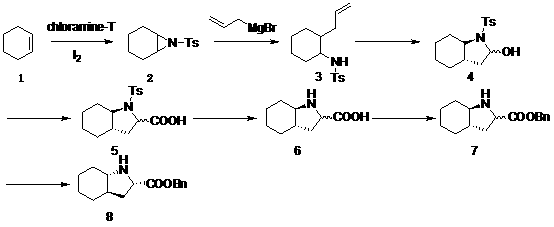
The invention relates to a method for preparing a trandolapril intermediate in the field of biomedicines, i.e. a method for synthesizing (2S, 3aR, 7aS)-octahydroindole-2-carboxyl benzyl ester. Firstly, cyclohexene and chloramine T are taken as initial raw materials to react so that cyclohexane ethylene imine is obtained; cyclohexane ethylene imine and allylmagnesium bromide are reacted with each other to obtain trans-N-(p-toluene sulphonyl)-2-(2-allyl)-cyclohexylamine which is peroxidized so as to obtain racemic N-protecting octahydroindole-2-carboxylic acid under the action of an oxidizing agent; after a protecting group on nitrogen is removed, racemic octahydroindole-2-carboxyl benzyl ester is obtained through esterification reaction; and the racemic octahydroindole-2-carboxyl benzyl ester is recrystallized and separated to obtain the trandolapril key intermediate (2S, 3aR, 7aS)-octahydroindole-2-carboxyl benzyl ester. According to the method, raw materials are low in cost and easy to obtain, the preparation process is environmental-friendly, the operation and post-treatment are simple, separation and purification are easy and the like.
Owner:SHANGHAI JIAO TONG UNIV
Residual chlorine standard substance, application of residual chlorine standard substance and residual chlorine tester correction or calibration method
InactiveCN103293121AColor/spectral properties measurementsMaterial electrochemical variablesIodineChloramine-T
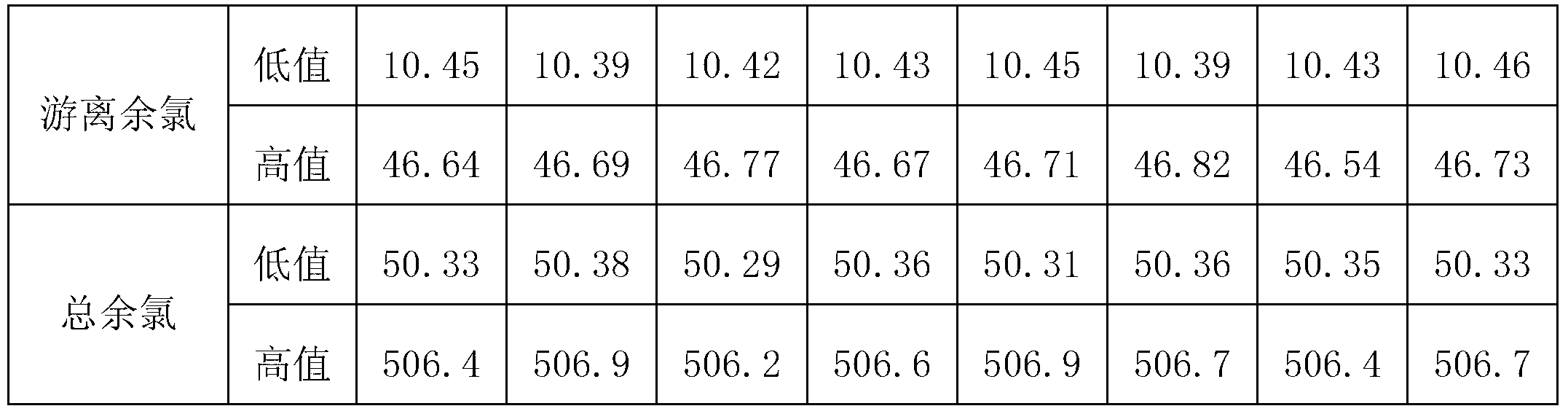
The invention provides a residual chlorine standard substance. The residual chlorine standard substance comprises the following two series: a free residual chlorine standard substance and a total residual chlorine standard substance, wherein the free residual chlorine standard substance comprises a low-value free residual chlorine standard substance and a high-value free residual chlorine standard substance; the concentration of the low-value free residual chlorine standard substance is 10+ / -1.0mg / L; the concentration of the high-value free residual chlorine standard substance is 50+ / -5.0mg / L; the total residual chlorine standard substance comprises a low-value total residual chlorine standard substance and a high-value total residual chlorine standard substance; the concentration of the low-value total residual chlorine standard substance is 50+ / -1.0mg / L; the concentration of the high-value total residual chlorine standard substance is 500+ / -10.0mg / L; a residual chlorine standard solution is prepared by taking an iodine standard solution and chloramine T as a reagent main body according to the required concentration. The invention also provides a residual chlorine standard substance testing method.
Owner:SHANGHAI INST OF MEASUREMENT & TESTING TECH
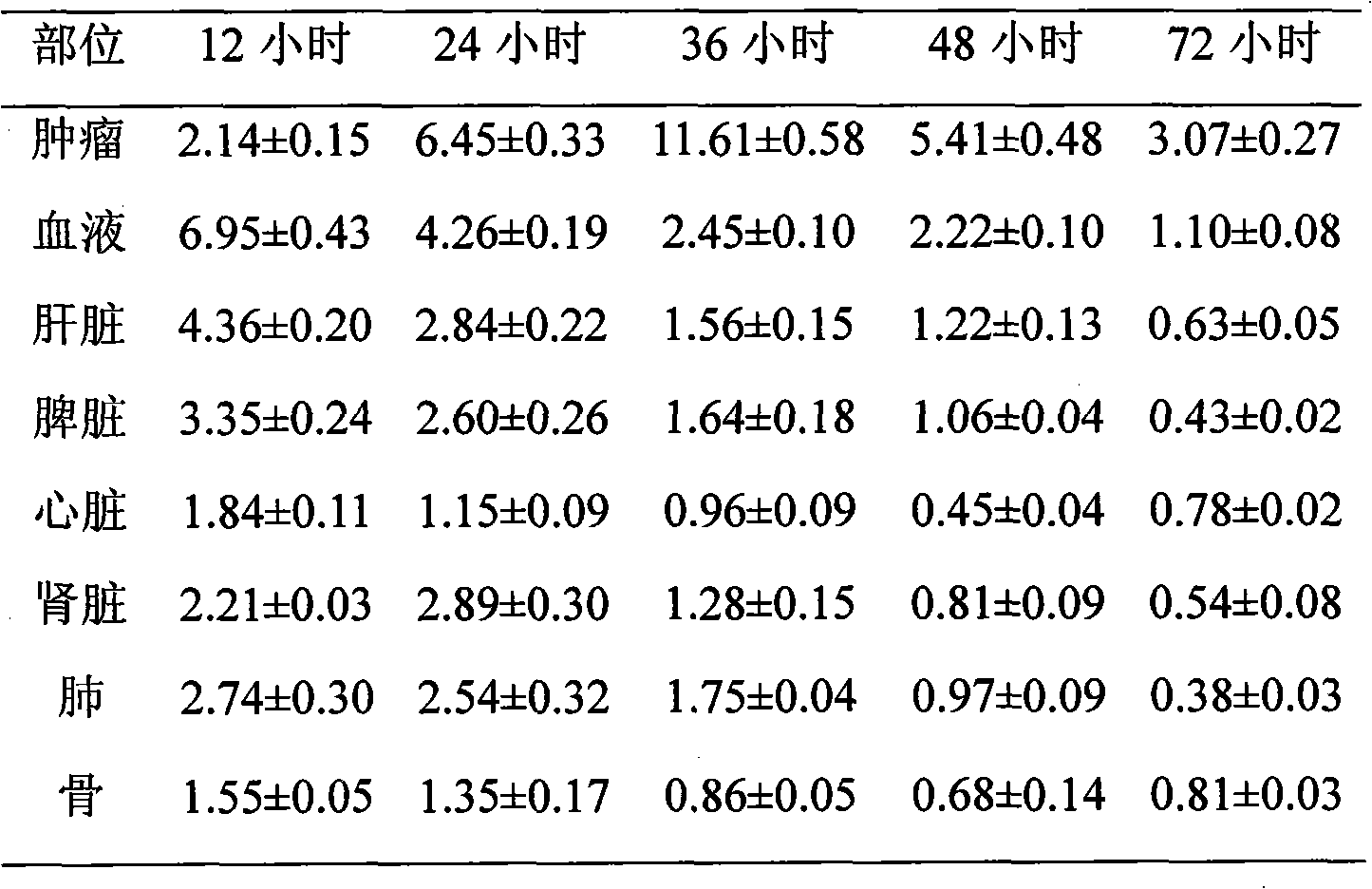
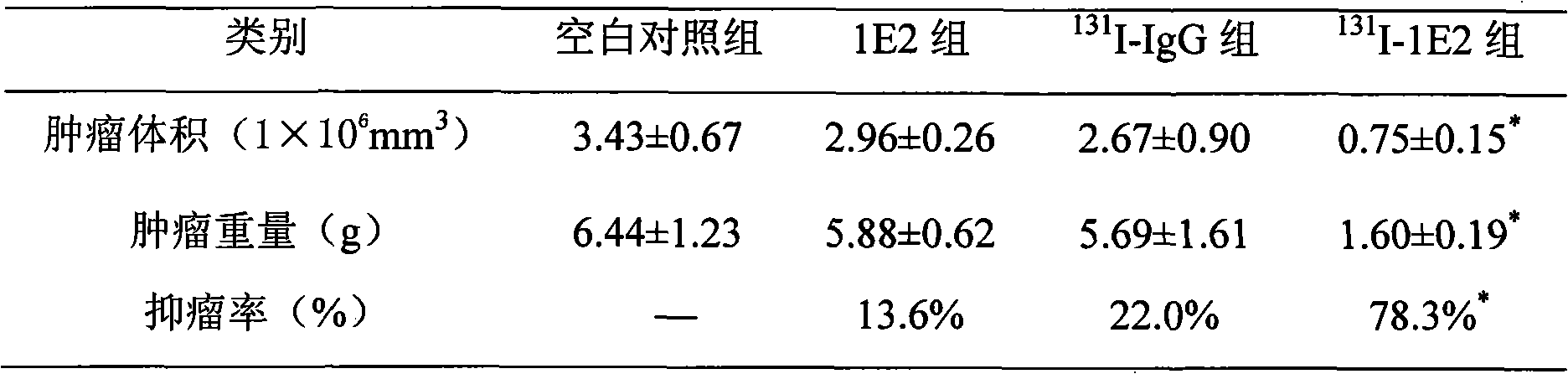
131I labeled anti-tumor humanized monoclonal antibody 1E2 and use thereof
InactiveCN101407545AImprove targetingGood inhibitory effectIn-vivo radioactive preparationsImmunoglobulins against animals/humansAntigenPhage antibodies
The invention provides an anti-lung cancer human resource monoclonal antibody 1E2 marked by <131>I. The human resource 1E2 antibody, Na <131>I and chloramine T are used as raw materials, and purified 1E2 antibody marked by the <131>I can be prepared by using the chloramine T method, wherein, the human source 1E2 antibody is obtained by taking paracanser lymph nodes of a non-small cell lung cancer patient, extracting total RNA in lymph node tissues, and then a phage antibody library containing the 1E2 antibody is obtained by using the constructing technology of the phage antibody library, and the phage antibody 1E2 is adsorbed by using a carbamyl phosphate synthetase antigen, and a human source 1E2 antibody which is specific to the carbamyl phosphate synthetase is finally obtained by washing, screening and amplifying. The invention applies radiation immunity treatment technology, combines advantages of radionuclide and anti-tumor cell human source monoclonal antibody to one, improves the targeting ability and curative effect of medicines to tumor cells, reduces side effects and poor immune reactions, and is wider in application range and suitable for treatments of the non-small cell lung cancer of each stage.
Owner:CHONGQING MEDICAL UNIVERSITY
Dental and oral formulation containing protease and preparation method thereof
The invention relates to the technical field of medical care, and provides a dental and oral formulation containing protease and a preparation method thereof. The main components of the formulation are chloramine-T and protease, and can be used in root canal flushing or decay removing of decayed teeth, dental whitening and small oral ulcer treatment and the like.
Owner:武汉伢典生物科技有限公司
Process for stain removal
InactiveUS20040102348A1Organic detergent compounding agentsSurface-active detergent compositionsChloramineSlurry
Chloramine-T has been used as a biocide for over one hundred years. Chloramine-T is also useful as a stain removal agent. This invention is concerned with a process whereby stains on a variety of substrates may be removed by treating a stained area of a substrate with a solution of Chloramine-T. In one embodiment a stained substrate is sprayed with a solution of Chloramine-T. The stained area may also be treated with a slurry or paste of Chloramine-T in order to effect stain removal. Solutions of Chloramine-T have the ability to remove undesirable stains without adversely affecting the substrate i.e. a piece of clothing.
Owner:SCHNEIDER CHARLES A +1
Injectable polypeptide hydrogel for local radiotherapy on tumor and preparation method of injectable polypeptide hydrogel
ActiveCN106215199AStable non-dispersed stateStrong growth inhibitory effectOrganic active ingredientsAerosol deliveryLocal radiotherapyPolyethylene glycol
The invention relates to injectable polypeptide hydrogel for local radiotherapy on tumor and a preparation method of the injectable polypeptide hydrogel. The injectable polypeptide hydrogel consists of polyethylene glycol and hydrophobic polytyrosine macromolecule block polymers in a self-assembled manner; a radiotherapy agent 131I is marked on a polytyrosine chain segment of hydrogel by using a chloramine T method, then loading of the radiotherapy agent can be completed, and the injectable polypeptide hydrogel for local radiotherapy can be prepared. The injectable polypeptide hydrogel provided by the invention has the characteristics of being high in radiotherapy agent loading rate, free of toxin, good in stability and degradability, easy to inject and the like. Specificity long-term local radiotherapy of gel on tumor is innovated, and compared with other radiotherapy preparations, the injectable polypeptide hydrogel has the advantages that injection treatment can be easily achieved, operation invasive implantation and seed source removal are avoided, radiation injury of non-tumor tissue can be avoided, and the pharmacological function lasts for a long time.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Preparation method of 1-bromoalkyne and 1-iodoalkyne
ActiveCN108658724AEasy to operateSimple post-processingOrganic compound preparationHalogenated hydrocarbon preparationSodium iodideLithium bromide
The invention discloses a preparation method of 1-bromoalkyne and 1-iodoalkyne. The preparation method comprises the following steps that terminal alkyne is adopted as a raw material, a chloramine salt and an iodine salt or a bromine salt is adopted as a halogenation system, and reaction is carried out in a solvent to synthesize a series of 1-bromoalkyne and 1-iodoalkyne type compounds. In the formula shown in the description, R in terminal alkyne is selected from chain-like alkyne, ring-shaped alkyne, olefin, ester group, cyanogroup, substituted phenyl group and heterocyclic arene; the chloramine salt is selected from one of chloramine B, chloramine T or o-chloramine T; the iodine salt is selected from one of sodium iodide, potassium iodide, ammonium iodide, lithium iodide or tetrabutylammonium iodide; the bromine salt is selected from one of sodium bromide, potassium bromide, lithium bromide, magnesium bromide, ammonium bromide or tetrabutylammonium bromide; the solvent is selected from one or is mixed by two of water, benzene, methylbenzene, 1, 4-dioxane, ethyl acetate, dimethyl sulfoxide, methanol, tetrahydrofuran, alcohol, isopropyl alcohol, N, N-dimethyl formamide, n-pentane,dichloromethane, petroleum ether, methyl tert-butyl ether, chloroform, n-hexane, carbon tetrachloride, n-butyl alcohol, 1,2-dichloroethane or acetonitrile. The preparation method disclosed by the invention has the beneficial effect that the application is wide.
Owner:ZUNYI MEDICAL UNIVERSITY
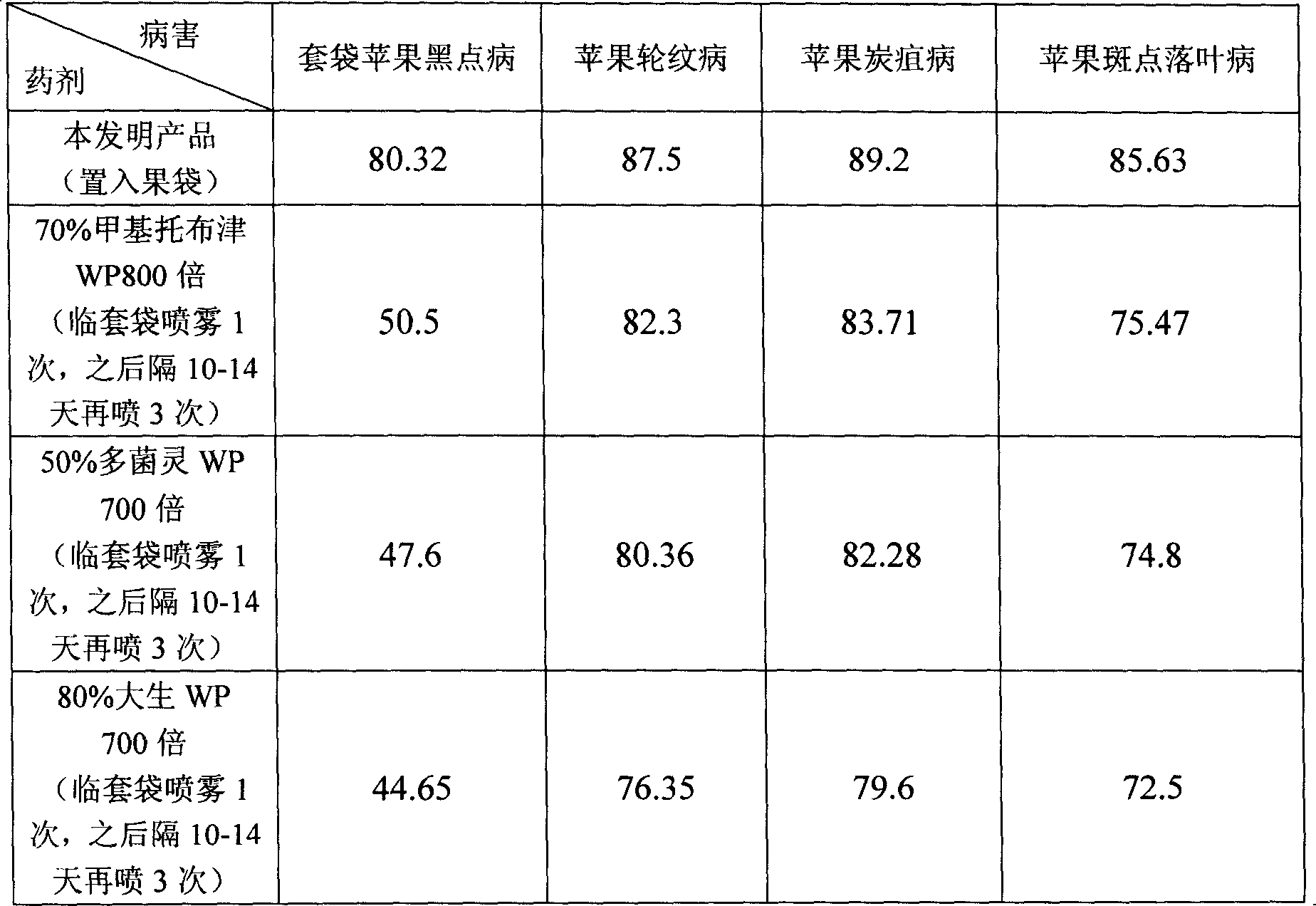
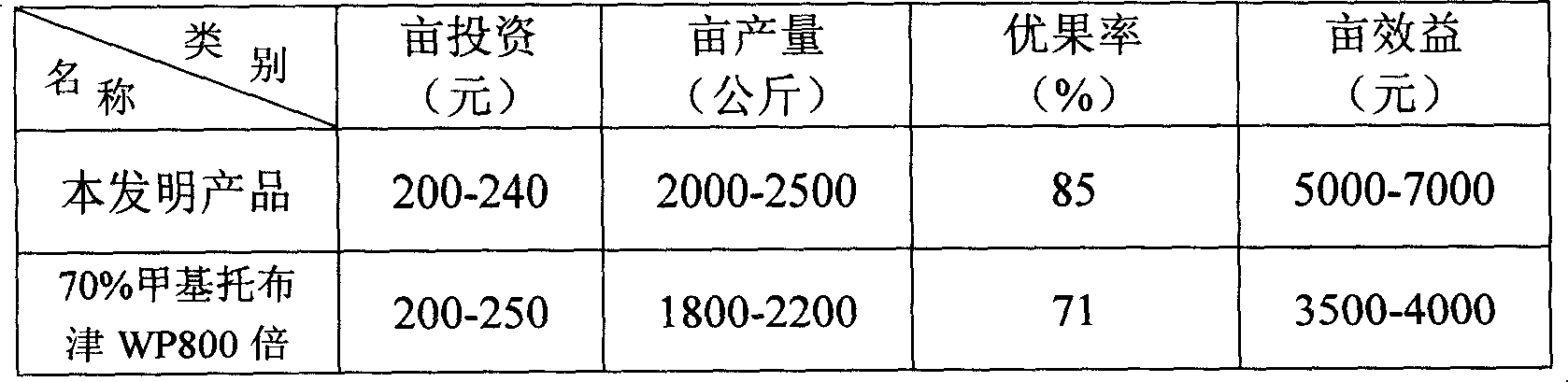
Medicine for treating and preventing bag fruit from disease and preparation method thereof
InactiveCN101142912ASecurity controlEffective controlBiocideDisinfectantsMethyl celluloseChloramine-T
The invention discloses a reagent for prevention and treatment of fruit disease of bagging fruit. The raw material of the reagent consists of chloramines T 50 percent to 80 percent, diatomite 11 percent to 25 percent, stearic acid 3 percent to eight percent, shellac 5 percent to 13 percent and hydroxypropyl methyl cellulose 1 percent to 4 percent. The invention also discloses the preparation method for the reagent which is as follows that three different particles are prepared with the formula according to different ratio so that the particle can release germicide at different time and disease prevention for a long time can be achieved. According to the characteristics of bagging fruit disease occurrence, the reagent provided by the invention changes traditional method for medicine application, not only reducing medicine application cost, but also improving prevention efficiency, increasing harvest level and commodity rate and reducing environmental pollution, moreover, win-win result can be achieved.
Owner:NORTHWEST A & F UNIV
Rapid determination method for bromine in oil-gas field water
InactiveCN102818780AMeet the needs of rapid determinationPreparing sample for investigationColor/spectral properties measurementsChloramineMass analyzer
The present invention discloses a rapid determination method for bromine in oil-gas field water, and relates to an analysis test method. The method comprises the following steps that: (1) a bromine standard curve is drawn; and (2) chloramine T is adopted to oxidize bromine ions in water into elemental bromine, and the bromine and phenol red form a purple tetrabromophenol red complex, wherein color intensity of the complex is deepened along with increase of the bromine concentration, such that a spectrophotometer is adopted to carry out colorimetric determination. According to the present invention, the spectrophotography is adopted to determine the bromine in the oil-gas field water, such that difficulties of high cost and high test environment requirements of application of ion chromatograph and inductively coupled plasma-mass spectrometer are overcome, and requirements of rapid determination of bromine in oil-gas field water are met.
Owner:WENGFU (GRP) CO LTD
Electroplating wastewater cyanide detection method and kit
ActiveCN104007108AMeet the testing requirementsSimple and fast operationMaterial analysis by observing effect on chemical indicatorHabitIsonicotinic acid
The invention discloses an electroplating wastewater cyanide detection method and kit. The method comprises the following steps: (1) firstly, reacting cyanide and chloramine-T in detected electroplating wastewater with the pH of 6.8-7.5 to generate cyan chloride; (2) secondly, reacting cyan chloride and isonicotinic acid, and hydrolyzing to generate 3-carboxyl pentene dialdehyde, so that the detected electroplating wastewater becomes red from orange red; and (3) thirdly, carrying out a dehydration condensation reaction on 3-carboxyl pentene dialdehyde and pyrazolone so as to generate a blue compound to perform colorimetric detection. The detection method provided by the invention conforms to the requirement for detecting cyanide in water by the national standard. The detection method of the kit provided by the invention is very simple and convenient to operate, can be implemented rapidly and conforms to the general operation habit of people. The actual consumption of a reagent in the kit provided by the invention is low, the kit can be used for detection for multiple times, the reagent cannot deteriorate at room temperature and can be used for a long time, and the kit meets the requirement on rapid field detection and is low in price and easy to popularize.
Owner:XIAMEN BAST CLEAN ENVIRONMENTAL TECH
Bone meal modified activated carbon and preparation method thereof
InactiveCN103566892ANot easy to disintegrateWell-developed pore structureOther chemical processesSodium metasilicateCarbonization
The invention relates to bone meal modified activated carbon which is prepared from the following raw materials in parts by weight: 80-90 parts of activated carbon, 40-50 parts of bone meal, 4-6 parts of aluminum sulfate, 8-10 parts of sodium metasilicate, 8-10 parts of paligorskite, 3-5 parts of copper sulfate, 7-9 parts of kieselguhr, 2-3 parts of chloramine-T, 4-6 parts of polyethylene glycol, 4-5 parts of modified kieselguhr and a right amount of water. According to the invention, the bone meal is subjected to high-temperature carbonization to form a shape having a developed pore structure and a large specific surface area, and the adsorption rate for heavy metal (such as Cu, Pb, Hg, Cr, Cd and the like) ions is 70-98%; the paligorskite and kieselguhr are further used, so that the effects of bacterium resistance, deodorization, moisture absorption, catalysis and binding property can be achieved, thus ensuring that the activated carbon is less prone to disintegrate; by adding the aluminum sulfate, water pollutants can be flocculated; and by adding the chloramine-T at the same time, sterilization and disinfection can be realized. The activated carbon provided by the invention is suitable for daily air purification and water purification, and has favorable application prospects.
Owner:BENGBU HUAFANG FILTER
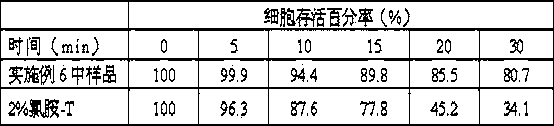
Method for labeling OxLDL-Ab by utilizing chloramine T
PendingCN106866819AGood repeatabilityApplicable to clinical imaging needsImmunoglobulins against animals/humansRadioactive preparation carriersSodium metabisulfiteChloramine-T
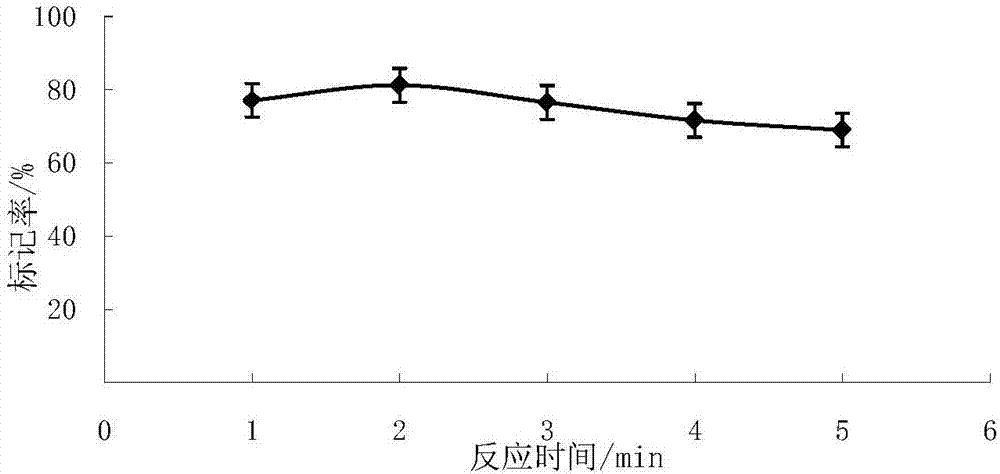
The invention belongs to the technical field of drug labeling and discloses a method for labeling OxLDL-Ab by utilizing chloramine T. The method comprises the following steps: (1) sequentially adding a PBS buffer, a NaI solution, an OxLDL-Ab solution and chloramine T in a reaction vessel, covering a bottle cap within 2 to 10 seconds and reacting for 1 to 5 minutes; (2) adding a sodium metabisulfite Na2S2O5 solution to the resultant reaction product so that the reaction is stopped, wherein the concentration of Na2S2O5 solution is 2.5 to 6 mg / ml, and the molar ratio of Na2S2O5 to chloramine T is 1.2 to 2.4; (3) leaching a PD-10 column with the PBS buffer until column equilibrium; (4) allowing the resultant reaction product obtained from the step (2) to pass the equilibrated PD-10 column obtained from the step (3). The method has the beneficial effects of high labeling rate, short labeling time, good stability, high radiochemical purity, and easiness in standardized operation.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
Glycosylated somatostatin analogue 123/131I-Gluc-KE108 and preparation method thereof
ActiveCN108017691AReduce false negative rateHigh sensitivityPeptide preparation methodsSomatostatin AnalogueChloramine-T
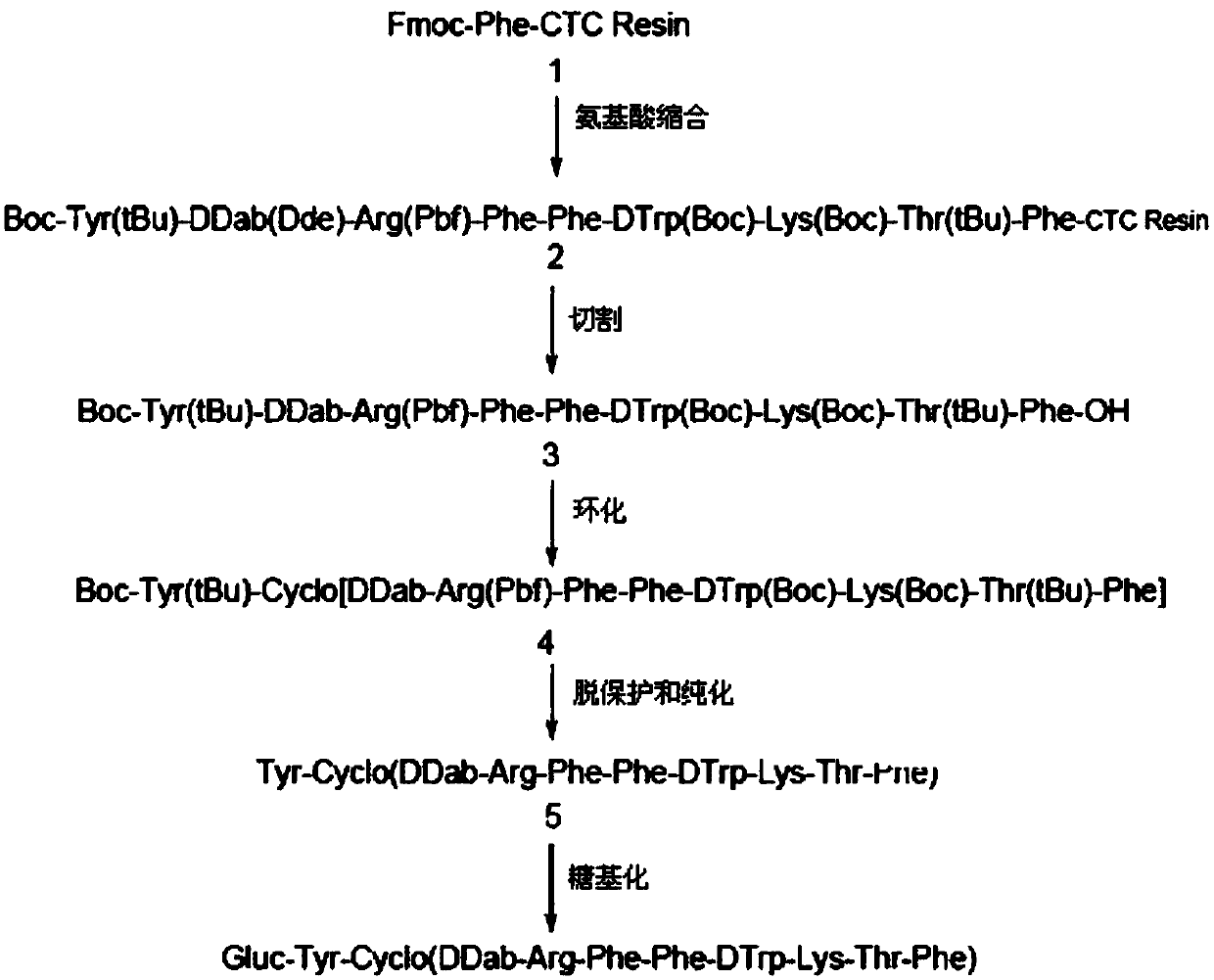
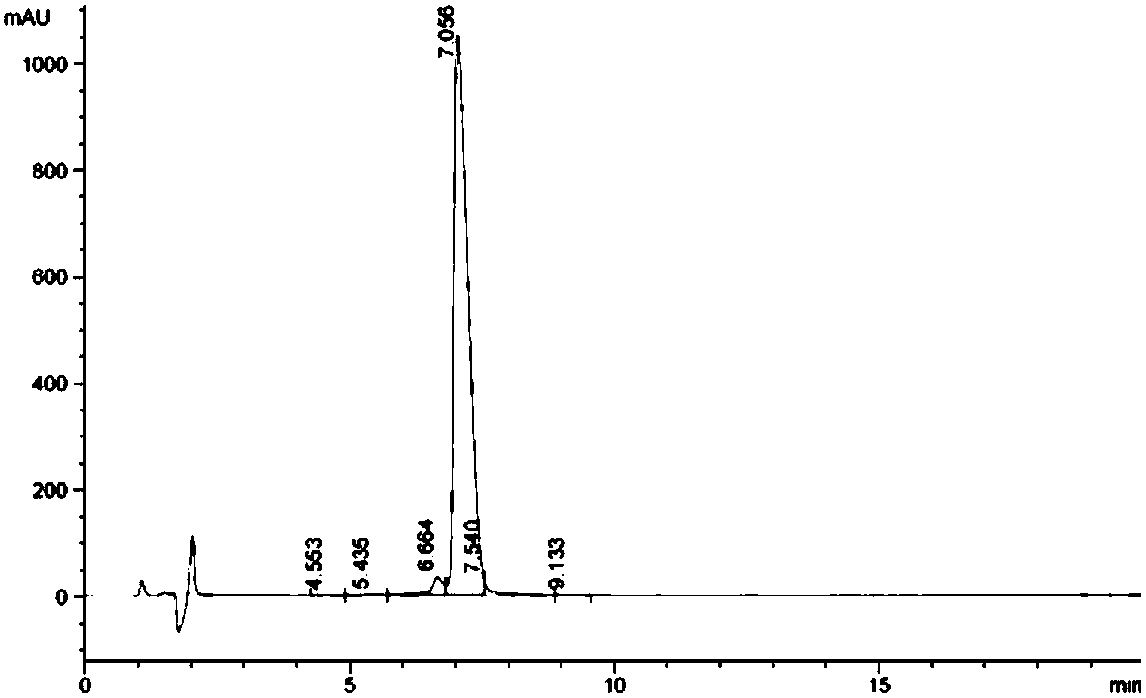
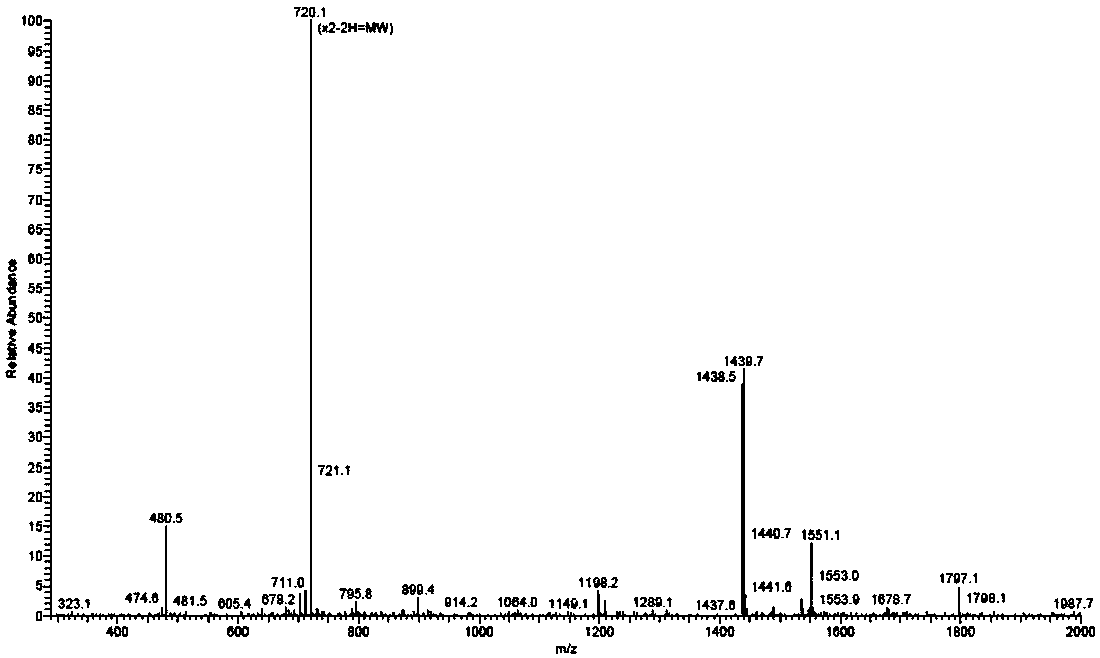
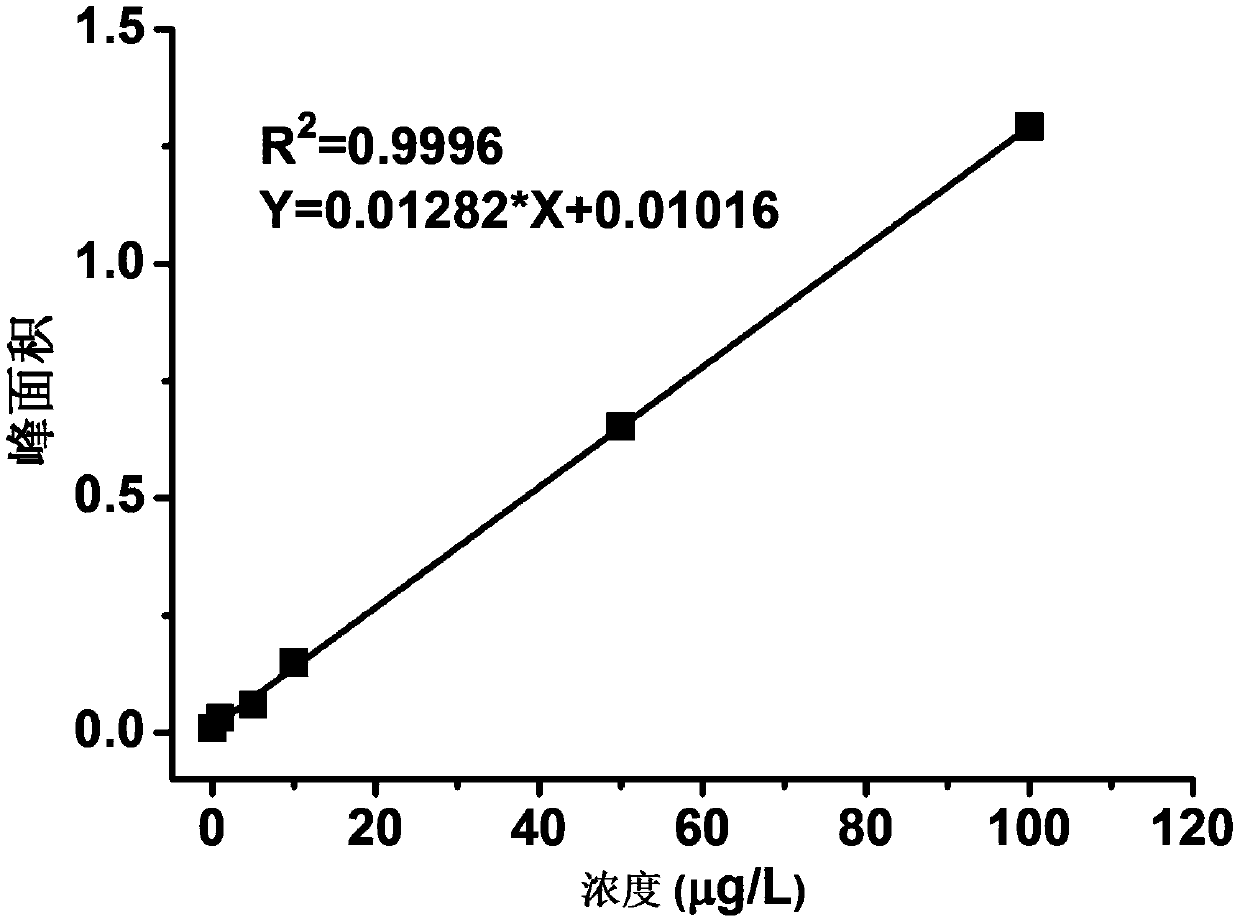
The invention belongs to the field of medicines, and concretely relates to a 123 / 131I-labeled novel glycosylated somatostatin analogue 123 / 131I-Gluc-KE108 and a preparation method thereof. The preparation method of the 123 / 131I-labeled novel glycosylated somatostatin analogue 123 / 131I-Gluc-KE108 comprises the following steps: (S1) synthesizing a novel glycosylated somatostatin analogue Gluc-KE108;and (S2) labeling the Gluc-KE108 with 123 / 131I, wherein step (S1) comprises (S1-1) amino acid condensation, (S1-2) removal of Dde from a polypeptide, (S1-3) resection of the polypeptide from resin, (S1-4) cyclisation of the polypeptide, (S1-5) acidolysis of the polypeptide and purification of an intermediate, and (S1-6) glycosylation and purification of the polypeptide; and in step (S2), the Gluc-KE108 is labeled with the 123 / 131I through adopting an Iodogen technology or a chloramine-T technology.
Owner:HTA CO LTD
Method for detecting cyanide content in electroformed gold product by headspace gas chromatography
ActiveCN111257494AEasy detectionEffective absorptionComponent separationProcess efficiency improvementOXALIC ACID DIHYDRATEO-Phosphoric Acid
The invention belongs to the technical field of gold product detection and particularly discloses a method for determining the cyanide content in an electroformed gold product by gas chromatography. The method comprises steps of 1) weighing the electroformed gold product as a test sample, and dissolving the test sample by using mixed reagent of hydrogen peroxide, sodium chloride and oxalic acid; 2) distilling out hydrogen cyanide through disodium ethylene diamine tetraacetate, tartaric acid and trichloroacetic acid under a boiling condition, and absorbing with sodium hydroxide solution to obtain cyanide aqueous solution for detection; and 3) adding 10mL of the sample into a headspace sample injection bottle containing phosphoric acid and chloramine T, determining the concentration of cyanide by headspace gas chromatography, and calculating the content of cyanide in the to-be-determined solution by calibrating a working curve. The method is advantaged in that the method can be used foraccurately measuring the content of cyanide in the electroformed gold product, fills up the technical blank that the cyanide in the product cannot be measured, and is convenient and quick in detectionprocess, accurate in detection result and good in consistency.
Owner:NANJING INST OF PROD QUALITY INSPECTION +1
Novel tumor marking method
InactiveCN110237277ATag implementationHigh sensitivityRadioactive preparation carriersVascular endothelial growth factorChloramine-T
Provided is a novel tumor marking method. By marking a vascular endothelial growth factor (VEGF) with radioactive iodine 131, 131I marking of VEGF antibodies is conducted through a chloramine-T method, and PB liquid is used for preparing chloramine-T solution and Na2S2O5 solution. A certain amount of VEGF antibodies are taken and placed in an EP tube, and the EP tube is placed in a refrigerator of 4 DEG C; a certain amount of Na131I solution, PB liquid and chloramine-T solution are added to the EP tube successively; obtained solution is immediately stirred through an electromagnetic stirrer for a while; 100 ml Na2S2O5 solution is quickly added to terminate the reaction. According to the novel tumor marking method, the vascular endothelial growth factor (VEGF) is marked with radioactive iodine 131, 131I marking of VEGF antibodies is conducted through a chloramine-T method, and the method is simple, convenient, accurate and efficient.
Owner:北京健平金星医疗器械有限公司
Use of piezoelectric transducers modified with metal oxide-based thin films for direct detection of amine derivatives in liquid media
InactiveUS20180080902A1High sensitivityHighly capableAnalysing fluids using sonic/ultrasonic/infrasonic wavesTesting organic contamination in waterLiquid mediumChloramine
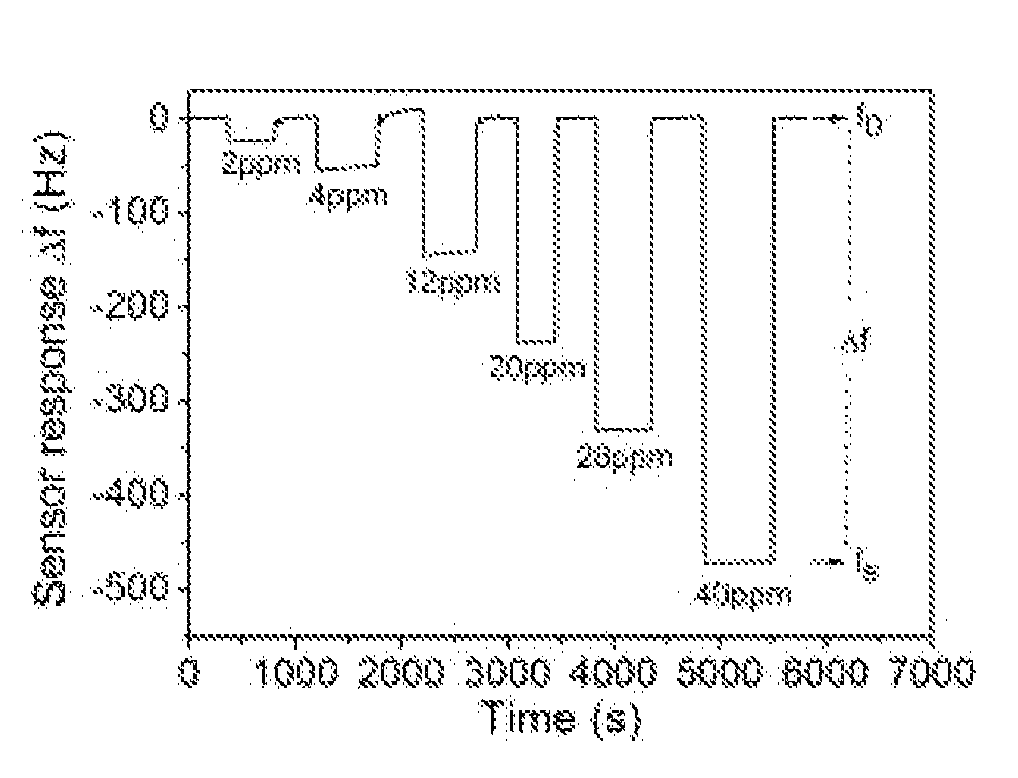
The invention discloses a chemical sensor device including a sensitive material formed as a thin film on the active side of a transducer substrate. The sensor uses vanadium oxide (V2O5) as a sensing material to detect amine and amine derivatives directly in liquid media. The developed sensor has been tested against selected amines as target analytes (such as triethylamine, butylamine, hexylamine and chloramine-T), organic chlorinated, non-chlorinated compounds, phenols, and a variety of pesticides which can be found in water and act as interferences. The sensor has exhibited sensitivity values of 12 Hz / ppm against chloramine-T and in the range of 1-2 Hz / ppm for the amines. The detection limit is 80 ppb for chloramine-T and below 1 ppm for the amines. The developed sensor does not respond to potentially interfering compounds.
Owner:TUBITAK
Trandolapril intermediate preparation method
ActiveCN104045593ALower synthesis costHigh yieldOrganic chemistryBulk chemical productionGrignard reagentAziridine
The invention relates to a trandolapril intermediate preparation method which is as follows: reacting cyclohexene and chloramines-T as starting materials to obtain cyclohexane aziridine; reacting the cyclohexane aziridine with an allyl Grignard reagent to obtain trans-N-p-toluenesulfonyl-2-(2-propenyl)-cyclohexylamine, obtaining trans D,L-N-p-toluenesulfonyl-octahydro-1H-indole-2-formic acid under the effect of an oxidizing agent, performing methyl esterification protection on free carboxyl to obtain trans D,L-N-p-toluenesulfonyl-octahydro-1H-indole-2-methyl formate, after removal of Ts protecting group connecting with nitrogen and hydrolysis, performing benzyl esterification reaction to obtain trans D,L-octahydro-1H-indole-2-benzyl formate, and then separating and resolving to obtain a trandolapril key intermediate (2S, 3aR, 7aS)-octahydro-1H-indole-2-benzyl formate. The trandolapril intermediate preparation method has the advantages of cheap and easily obtained materials, environmentally friendly preparation process and simple operation and post-treatment.
Owner:SHANGHAI JIAO TONG UNIV
Detection test paper for pyrethroid pesticide residues in foods
InactiveCN106442507AQuick checkEasy to operateMaterial analysis by observing effect on chemical indicatorPhosphateNiacin
The invention discloses detection test paper for pyrethroid pesticide residues in foods. The detection test paper is prepared by immersing filter paper in a mixed solution and drying, wherein the mixed solution is prepared from the following components: 3g / L to 70g / L of phosphate buffering solution, 10g / L to 100g / L of chloramine T solution, 6g / L to 120g / L of isonicotinic acid-barbituric acid color developing agent and 5mL to 60mL of dichloromethane-methanol mixed solution, and a solvent which is de-ionized water. The volume ratio of dichloromethane to methanol in the dichloromethane-methanol mixed solution is (80 to 110) to 1. The phosphate buffering solution is obtained by mixing citric acid and phosphate. The mass ratio of isonicotinic acid to barbituric acid in the isonicotinic acid-barbituric acid color developing agent is (1 to 5) to 1. Before the detection test paper is used, the foods are crushed and sieved; then ethyl acetate is added for extracting; centrifuging is carried out and then a proper amount of supernatant is taken; the supernatant is volatilized to be nearly dry and then is dissolved with ethanol to obtain a sample to be detected. The detection test paper provided by the invention is used for detecting the pyrethroid pesticide residues in the foods and operation is simple and rapid; rapid detection on the pyrethroid pesticide residues in agricultural and sideline products is realized, and the detection test paper is suitable for rapid detection on site and in primary laboratories.
Owner:BEOSON JIANGSU FOOD SAFETY TECH CO LTD
Method for quantitatively detecting thiocyanate in milk and milk products
InactiveCN104165854AMeet the needs of on-site rapid detectionEasy to operateColor/spectral properties measurementsSodium thiocyanatePotassium
The invention provides a method for quantitatively detecting thiocyanate in milk and milk products. The method is used for quantitatively determining content of thiocyanate in milk and milk products by spectrophotometry. The method is simple to operate, relatively low in detection limit, short in detection time, high in accuracy, good in reproducibility, relatively high in recovery rate and suitable for fast detection of thiocyanate. The method comprises the following steps: (1) processing a sample; and (2) detecting the sample, wherein reagents used by the method comprise a sodium thiocyanate reagent A, a sodium thiocyanate reagent B, a sodium thiocyanate reagent C and a sodium thiocyanate reagent D; the sodium thiocyanate A is an acetic acid solution, and a volume ratio of water to acetic acid in the acetic acid solution is 10 to 50; the sodium thiocyanate reagent B is an anhydrous di-potassium hydrogen phosphate solution with a mass fraction of 5%-30%; the sodium thiocyanate reagent C is a chloramines T solution with a mass fraction of 1%-10%; the sodium thiocyanate reagent D is a mixed solution of isonicotinic acid with a mass fraction of 1%-10%, barbituric acid with a mass fraction of 1%-5% and sodium hydroxide with a mass fraction of 1%-10%.
Owner:BEIJING ZHIYUNDA TECH CO LTD
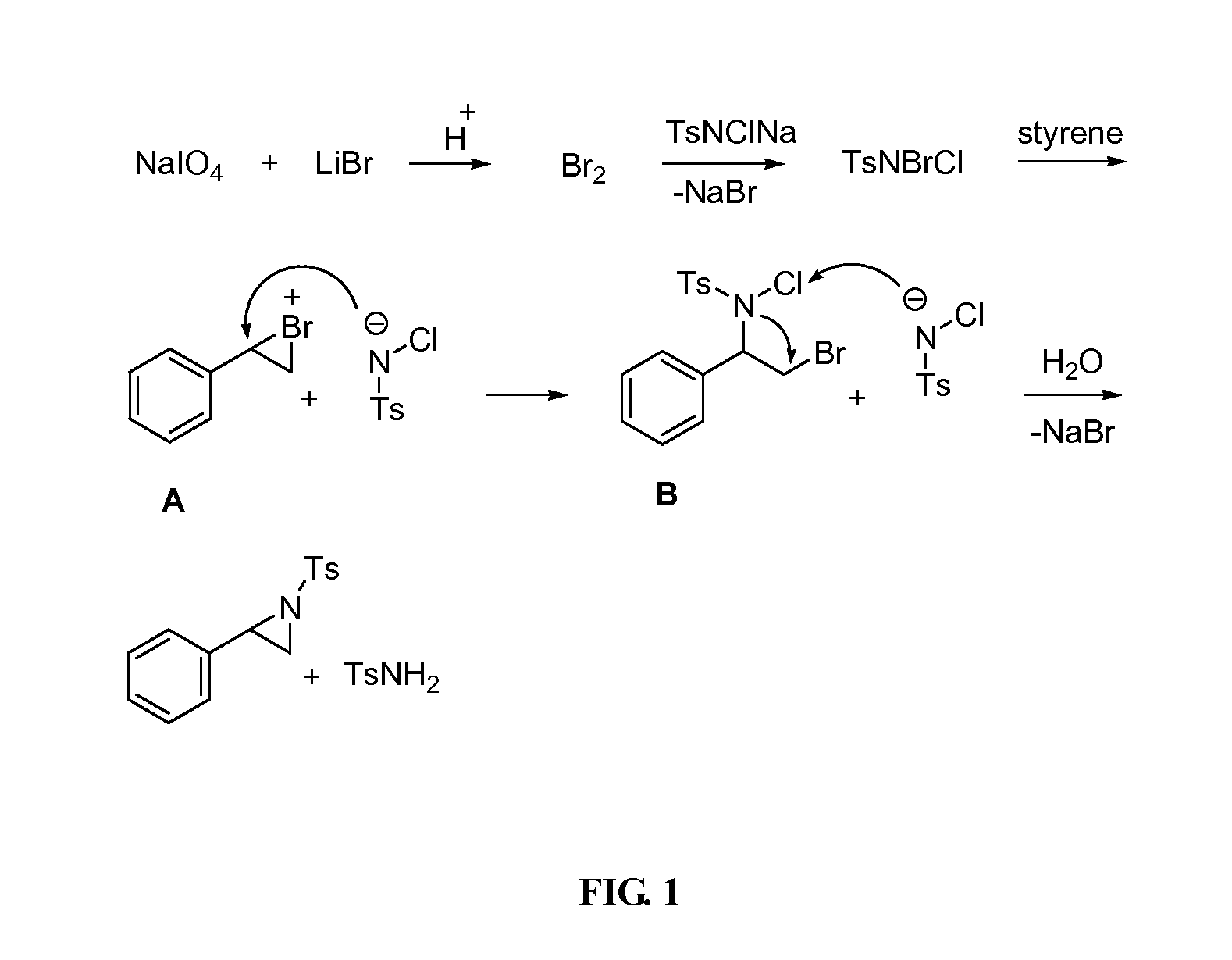
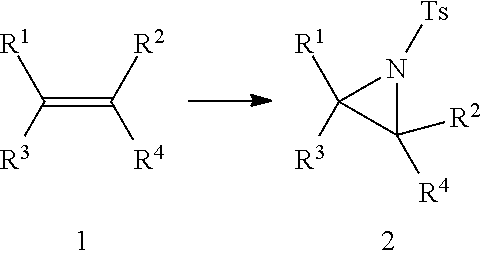
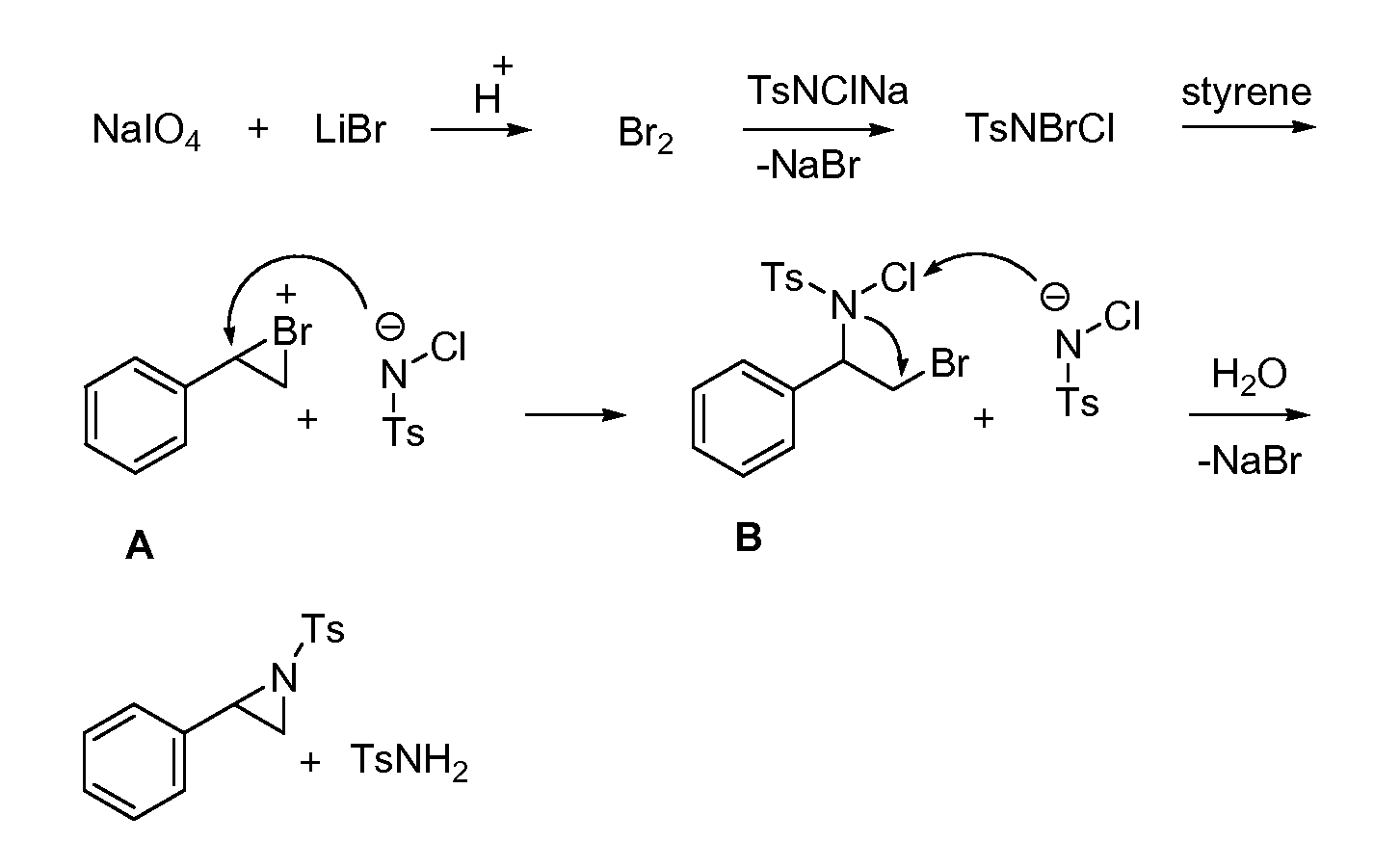
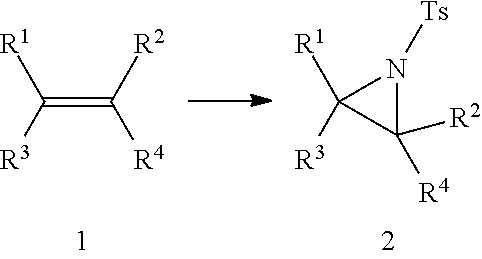
Aziridination of Olefins
InactiveUS20120215011A1Simple and inexpensiveSimple and inexpensive and environmentally benignOrganic chemistryAziridineChloramine-T
A process for aziridination of olefins using NaIO4 / alkali metal bromide / H+ / Chloramine-T combination in presence of dipolar aprotic solvent under ambient conditions to obtain aziridines is disclosed.
Owner:COUNCIL OF SCI & IND RES
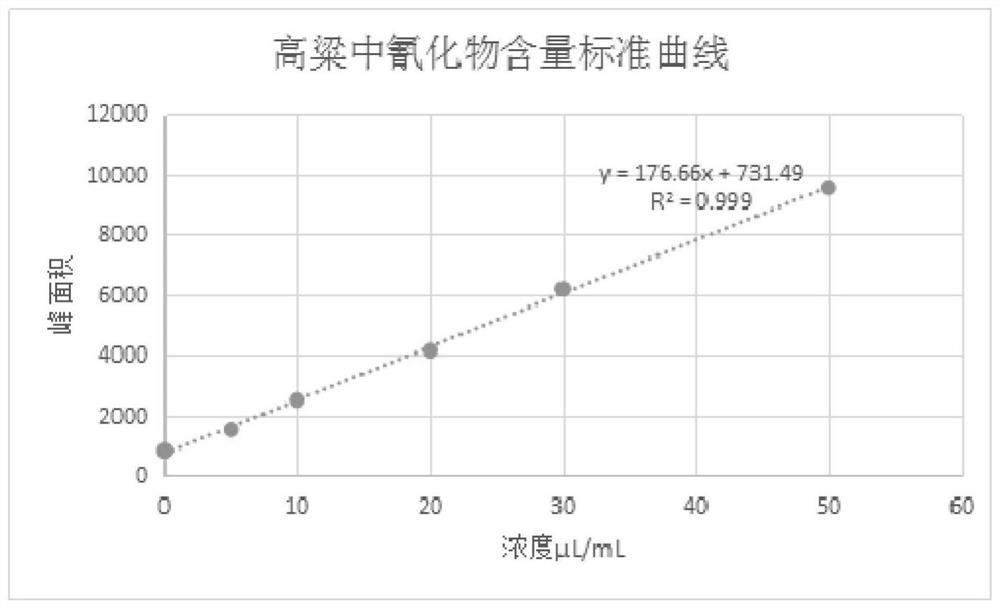
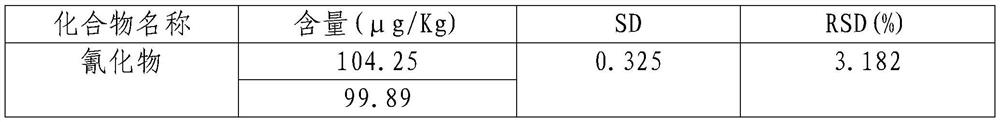
Rapid detection method for cyanide in sorghum
PendingCN112881548ALow detection valueImprove accuracyComponent separationBiotechnologyCyanide level
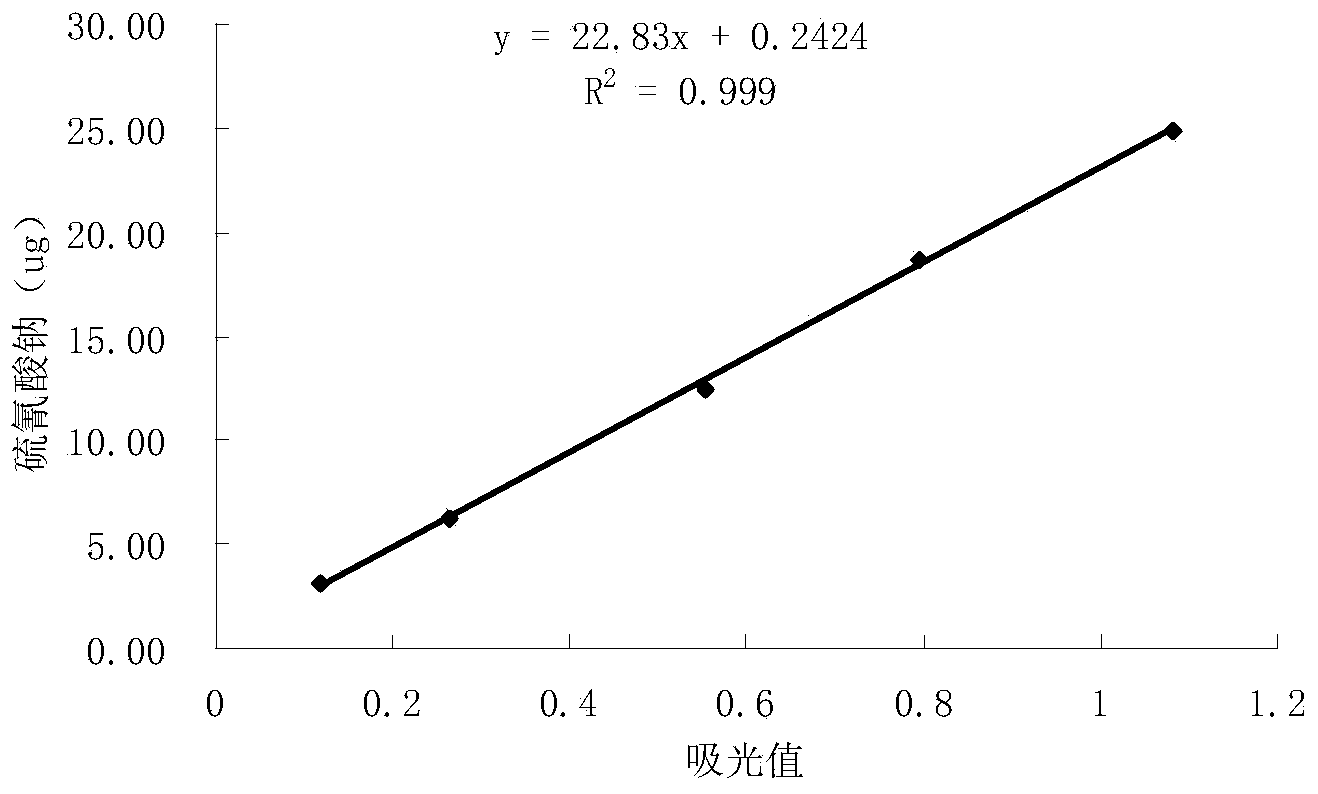
The invention belongs to the field of food analysis, and provides a rapid detection method of cyanide in sorghum. The method comprises the following steps of: directly adding a crushed sample into a headspace sample introduction bottle; ultrasonically extracting cyanide in sorghum by using ultrapure water; performing still standing in an ice-water bath for 5 minutes; adding a phosphate buffer solution and chloramine T under the condition of the ice-water bath, immediately performing covering and uniformly mixing; performing detection through gas chromatography-headspace chromatography, adopting a sample injection mode with the split ratio being 50: 1; performing quantification through using a standard curve method; and finally, calculating the content of the cyanide in the sorghum. According to the method, a chromatographic column used for gas chromatography-headspace chromatography detection is an HP-5 chromatographic column, and the specification of the chromatographic column is 30 m*0.32 mm*0.25 mu m; and a detection result shows that R2 is equal to 0.999, the recovery rate is 89.5%-108.9%, the precision RSD is equal to 3.182%, and the detection limit is 3 [mu] g / L. The detection method has the advantages of good reproducibility, high precision and high sensitivity, and is suitable for detecting cyanide in sorghum.
Owner:济南趵突泉酿酒有限责任公司
Aziridination of olefins
InactiveUS8558010B2Simple and inexpensive and environmentally benignOrganic chemistryAziridineChloramine-T
A process for aziridination of olefins using NaIO4 / alkali metal bromide / H+ / Chloramine-T combination in presence of dipolar aprotic solvent under ambient conditions to obtain aziridines is disclosed.
Owner:COUNCIL OF SCI & IND RES
Kit and method for rapidly detecting thiocyanate in milk and milk product
InactiveCN104165890AQuick checkMeet the needs of rapid detectionMaterial analysis by observing effect on chemical indicatorSodium thiocyanateMonopotassium phosphate
The invention provides a kit and a method for rapidly detecting thiocyanate in milk and a milk product, which can rapidly detect the content of thiocyanate in the milk and the milk product, is high in sensitivity, short in detection time and good in repeatability and can meet the requirement of enterprises and supervision and law enforcing departments such as quality supervision, and industry and commerce on in-situ rapid detection. The kit comprises a sodium thiocyanate reagent A, a sodium thiocyanate reagent B, and a sodium thiocyanate reagent C; the sodium thiocyanate reagent A is anhydrous monopotassium phosphate solution with the mass fraction of 5 to 30 percent; the sodium thiocyanate reagent B is chloramine T solution with the mass fraction of 1 to 10 percent; the sodium thiocyanate reagent C is a mixed solution of isonicotinic acid, barbituric acid and sodium hydroxide, the mass fraction of isonicotinic acid is 1 to 10 percent, the mass fraction of barbituric acid is 1 to 5 percent, and the mass fraction of sodium hydroxide is 1 to 10 percent.
Owner:BEIJING ZHIYUNDA TECH CO LTD
I<131> labeled anti-human neuropilin receptor-2 monoclonal antibody E4 and application thereof
InactiveCN107412795AImprove targetingImprove Molecular Imaging EfficiencyPharmaceutical delivery mechanismRadioactive preparation carriersTherapeutic effectIodination reaction
The invention relates to an I<131> labeled anti-human neuropilin receptor-2 (NRP-2) monoclonal antibody E4. The antibody E4 is labeled by I<131> through a chloramine T labeling method. The I<131>-E4 is produced through the following steps: 1) preparing the antibody E4, NaI<131>, and PBS being 7.4-7.6 in pH value, and adding the components into an EP tube, adding newly-prepared chloramine T and rapidly vibrating and uniformly mixing the liquid, performing a reaction at room temperature, and adding a newly-prepared reduction agent, sodium metabisulfite, to terminate the iodination reaction; 2) moving out the reaction liquid, and measuring the labeling rate of a labeled product through TLC, normal saline being a developing solvent; 3) treating the reaction liquid on a Sephadex G25 column to purify the reaction liquid with PBS being about 7.4 in pH value as an eluting solution to prepare I<131>-E4. Advantages of the radionuclide are integrated with the antitumor cell humanized monoclonal antibody, so that the targeting property and molecular imaging effect of the medicine are improved. The antibody E4 can increase the early-stage diagnosis rate and monitor treatment effect of positive tumor expressed by the NRP-2, has a wider available range and is suitable for imaging positive tumors expressed by the NRP-2 in all stages.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
Lossless tearing-off method for painting heart of ancient writing/painting
InactiveCN106274260ASolve empty drumSolve the problem of warping and other diseasesPainting preservationAlpha-amylaseChloramine-T
The invention discloses a lossless tearing-off method for a painting heart of an ancient writing / painting. According to the method, the ancient writing / painting to be torn off is processed with an alpha-amylase aqueous solution with the mass fraction being 0.025%-0.2% firstly, then supporting paper and the painting hearth are separated naturally, the painting hearth is processed with a chloramines-T aqueous solution with the mass fraction being 0.5%-2% after the supporting paper is torn off, alpha-amylase left on the painting hearth loses activity, and the problems that new paste cannot be pasted to the writing / painting or the viscosity of the paste is not enough after the ancient writing / painting is torn off with the alpha-amylase, and consequently the mounted writing / painting bulges or warps or the like after being placed for a period of time are solved. According to the method, operation is easy, convenient and safe, the strength, color, durability and the like of the ancient writing / painting are not influenced, and re-mounting of the writing / painting is not affected either.
Owner:SHAANXI NORMAL UNIV
Method for preparing large granular chloramine T based on cooling crystallization
ActiveCN103772242AComplete appearancePromote growthOrganic chemistryOrganic compound preparationChloramineChloramine-T
The invention discloses a method for preparing large granular chloramine T based on cooling crystallization. The method comprises the following steps: (1) adding a chloramine T crude product to ethanol water, heating, stirring ad dissolving; (2) under a stirring condition, cooling the solution obtained in the step (1) till devitrifying and getting turbid, adding chloramine T seed crystals, growing the grain, and cooling to 8-12 DEG C, thus obtaining crystallization suspension liquid; and (3) filtering the crystallization suspension liquid obtained in the step (2), and drying so as to obtain the large granular chloramine T. The method is simple to operate; conditions are easy to control; the obtained product is short in period and relatively high in purity; the prepared chloramine T crystal product is large in particle, uniform in particle size and high in yield, and the appearance of the crystal is complete.
Owner:天津普恒康泰科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com