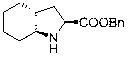
Method for preparing trandolapril intermediate
A technology for trandolapril and intermediates, which is applied in the field of new preparation of key intermediates, can solve the problems of unfavorable environmental protection and large-scale production, high cost of target products, expensive catalysts, etc., and achieve low cost, high yield and easy separation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
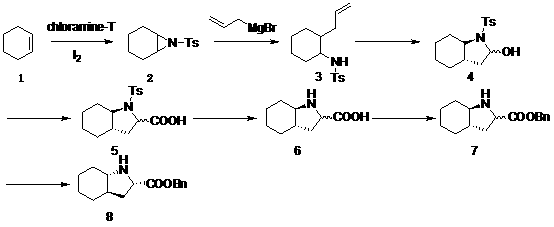
[0037] (1) Cyclohexaneziridine (hereinafter referred to as compound 2 )Synthesis
[0038] Chloramine T (500 g, 1.775 mol), cyclohexene (164 g, 2.0 mol), iodine (28.2 g, 0.11 mol) and benzyltrimethylammonium bromide (20.4 g, 0.088 mol) were added to 1000 mL tetrahydrofuran and 500 mL of water in a mixed solvent, react at room temperature for 24 h, distill off THF, extract the aqueous phase with ethyl acetate (500 mL×3), combine the organic phases, dry over anhydrous sodium sulfate, filter with suction, and remove the solvent by rotary evaporation , the product was recrystallized from ethanol to obtain white crystals (260 g, 58 %).
[0039] (2) trans-N-p-toluenesulfonyl-2-(2-propenyl)-cyclohexylamine (hereinafter referred to as compound 3 )Synthesis
[0040] Dissolve cyclohexaneziridine (25.1 g, 0.1 mol) and copper bromide (2.23 g, 0.01 mol) in 200 mL of ether, and allylmagnesium bromide (29 g, 0.2 mol) at -40 °C Slowly added dropwise and stirred overnight. The reaction was...
Embodiment 2
[0054] (1) Cyclohexaneziridine (hereinafter referred to as compound 2 )Synthesis
[0055] Chloramine T (500 g, 1.775 mol), cyclohexene (164 g, 2.0 mol), iodine (28.2 g) and benzyltrimethylammonium chloride (16.5 g, 0.088 mol ) were added to 1000 mL tetrahydrofuran and 500 mL In a mixed solvent of water, react at room temperature for 24 h. The tetrahydrofuran was evaporated, the aqueous phase was extracted with ethyl acetate (500 mL×3), the organic phases were combined, dried with anhydrous sodium sulfate, filtered with suction, and the solvent was removed by rotary evaporation, and the product was recrystallized with ethanol to obtain white crystals (270 g, 60%).
[0056] (2) trans-N-p-toluenesulfonyl-2-(2-propenyl)-cyclohexylamine (hereinafter referred to as compound 3 )Synthesis
[0057] Dissolve cyclohexaneziridine (25.1 g, 0.1 mol) and copper bromide (2.23 g, 0.01 mol) in 200 mL of ether, and allylmagnesium bromide (58 g, 0.4 mol) at -50 °C Slowly added dropwise and s...
Embodiment 3
[0071] (1) Cyclohexaneziridine (hereinafter referred to as compound 2 )Synthesis
[0072] Add 1000 mL of acetonitrile and 500 mL In a mixed solvent of water, react at room temperature for 24 h. Acetonitrile was evaporated, the aqueous phase was extracted with ethyl acetate (500 mL×3), the organic phases were combined, dried with anhydrous sodium sulfate, filtered with suction, and the solvent was removed by rotary evaporation, and the product was recrystallized with ethanol to obtain white crystals (275 g, 61%).
[0073] (2) trans-N-p-toluenesulfonyl-2-(2-propenyl)-cyclohexylamine (hereinafter referred to as compound 3 )Synthesis
[0074] Dissolve cyclohexaneziridine (25.1 g, 0.1 mol) and copper bromide (2.23 g, 0.01 mol) in 200 mL tetrahydrofuran, and allylmagnesium bromide (58 g, 0.4 mol) at -50 °C Slowly added dropwise, stirring overnight. The reaction was quenched with water, extracted with ethyl acetate (250 mL×3), the organic phases were combined, dried over anhydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com