Glycosylated somatostatin analogue 123/131I-Gluc-KE108 and preparation method thereof
A 131i-gluc-ke108, gluc-ke108 technology, applied in the preparation methods of peptides, chemical instruments and methods, organic chemistry and other directions, can solve problems such as non-targeting and false negatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
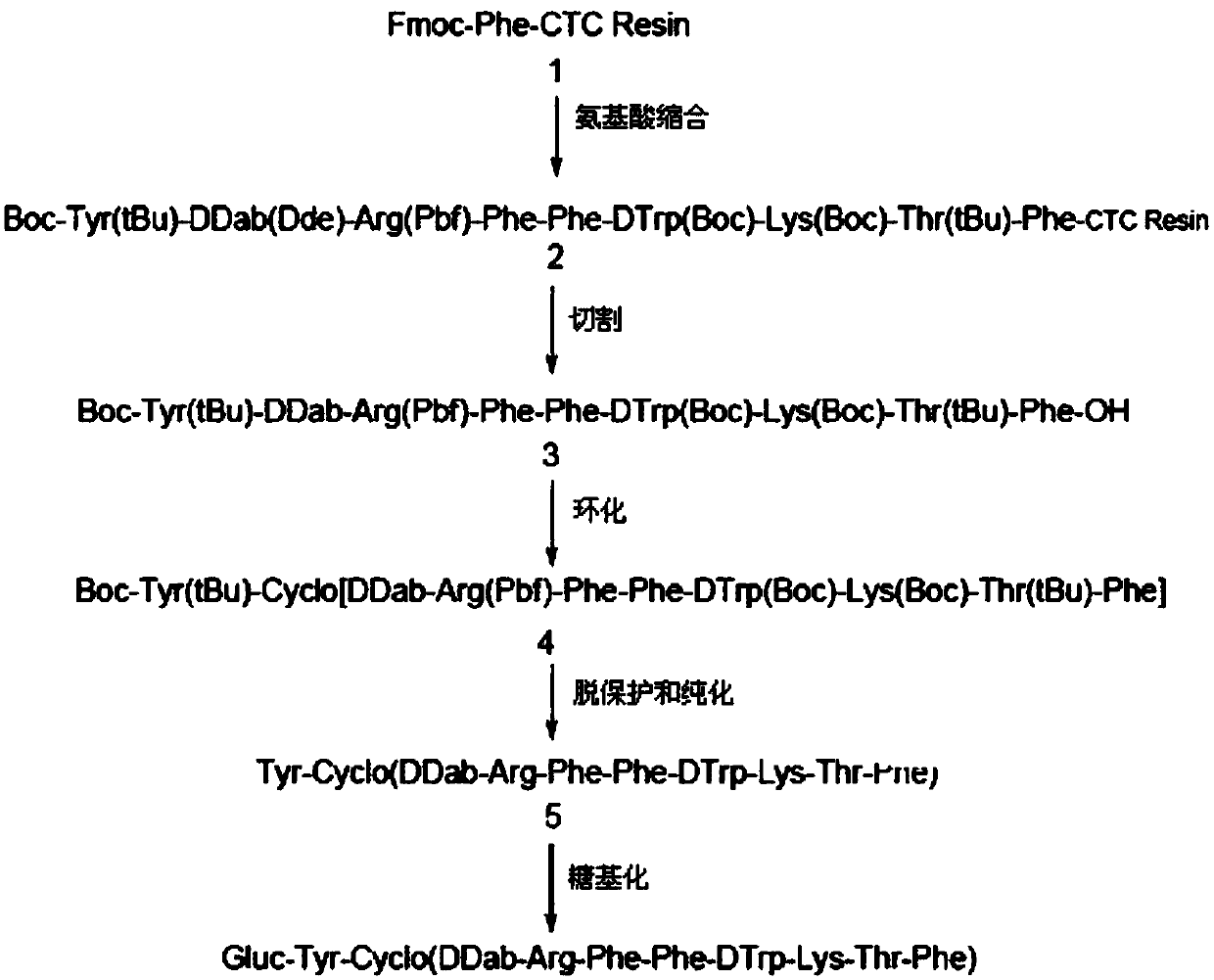
[0041] Synthesis of Gluc-KE108
[0042] The specific synthetic route of Gluc-KE108 is attached figure 1 As shown, the specific steps are as follows:
[0043] (S1-1) Condensation of amino acids
[0044] Weigh 0.71 g (0.3 mmol) of Fmoc-Phe-CTC Resin, add it to the polypeptide reactor, swell with DMF for 1 h, then add 20% piperidine and react at room temperature for 30 min to remove Fmoc, wash the resin 3 times with DMF, and then add 358mgFmoc-Thr(tBu)-OH, 324mg HBTU, 200μL NMM, after reacting for 50 minutes, it was detected that ninhydrin was negative, filtered and washed to obtain Fmoc-Thr(tBu)-Phe-CTC Resin, repeat the above operation and add the corresponding amino acids in turn: Fmoc- Lys(Boc)-OH(422mg), Fmoc-D-Trp(Boc)-OH(474mg), Fmoc-Phe-OH(349mg), Fmoc-Phe-OH(349mg), Fmoc-Arg(Pbf)-OH (584mg), Fmoc-D-Dab(Dde0-OH (0.454mg), Boc-Tyr(tBu)-OH (304mg), made Boc-Tyr(tBu)-DDab(Dde)-Arg(Pbf)-Phe -Phe-DTrp(Boc)-Lys(Boc)-Thr(tBu)-Phe-CTCResin.
[0045] (S1-2) De-Dde of polypept...
Embodiment 2
[0057] Gluc-KE108 was tested by Iodogen method 123 / 131 I mark:
[0058] The marked tube was pre-coated with 50 μg lodogen, and sequentially added 50 μL of 1 mg / mL Gluc-KE108 in dimethyl sulfoxide solution, 10 μL of 18.5-37 MBq Na 123 / 131 I, shaking reaction for 10 minutes, dissolved with physiological saline. Then use HPLC method or thin-layer chromatography to analyze its labeling rate, and after the product is separated and purified, use HPLC to analyze its radiochemical purity.
Embodiment 3
[0060] Using chloramine-T method (CH-T method) to Gluc-KE108 123 / 131 I mark:
[0061] Dissolve 50 μg Gluc-KE108 in 80 μL 0.5mol / L pH 7.4 phosphate buffer, then add 10 μL 18.5-37MBq Na 123 / 131 I, finally add 10 μL of 10 mg / mL chloramine-T solution. Gently shake the mixture at 20°C for 1-2min, then add 50μL pH7.4 4.0mg / mL sodium metabisulfite solution to stop the reaction. Then use HPLC method or thin layer chromatography to analyze its labeling rate, and after the product is purified, use HPLC to analyze its radiochemical purity.
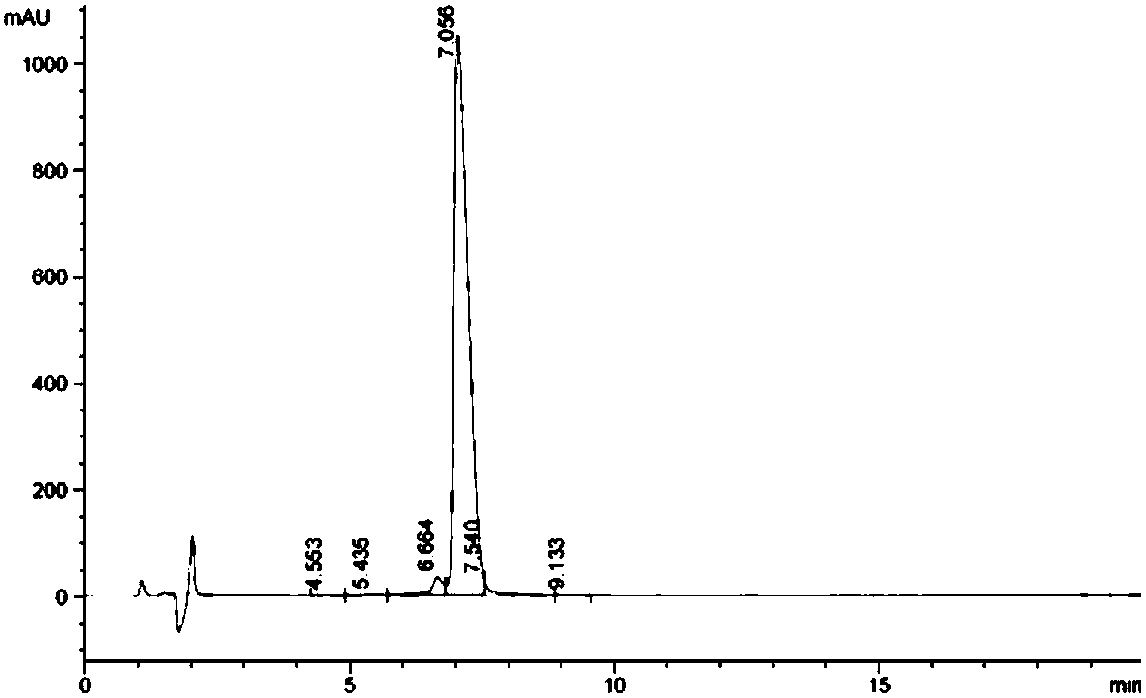
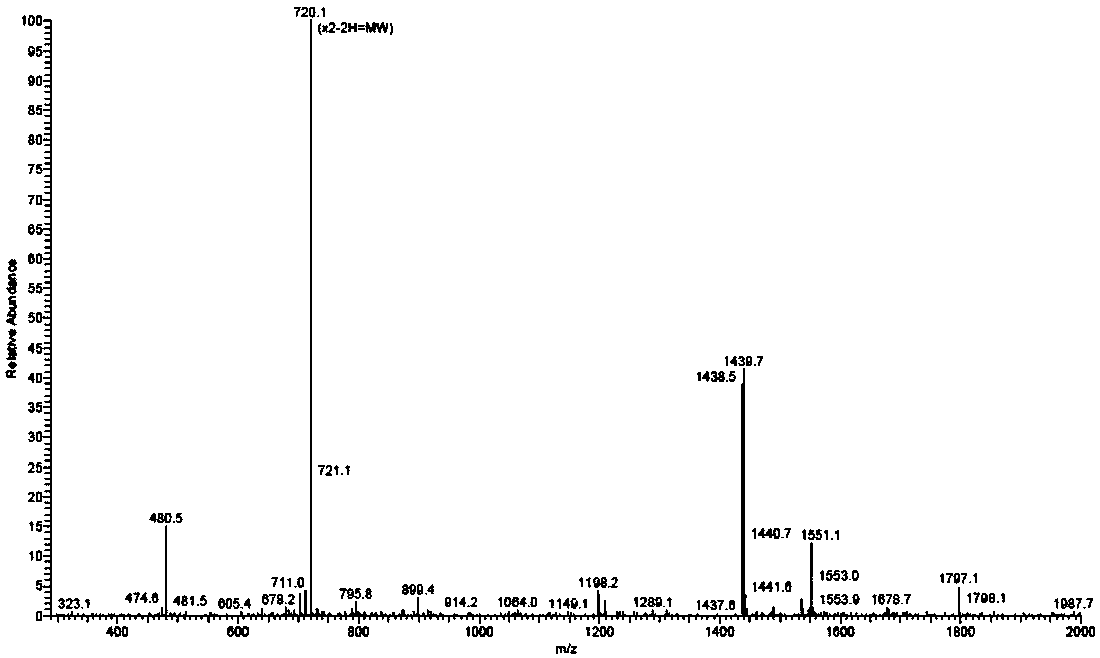
[0062] In summary, 16.3 mg of Gluc-KE108, i.e. Gluc-Tyr-Cyclo (DDab-Arg-Phe-Phe-DTrp-Lys-Thr-Phe), was synthesized by Fmoc solid-phase synthesis, and the purity was 95.2 mg by HPLC analysis. %, through mass spectrometry, it can be known that m / z is 720.1 ((M+2H) / 2) and 1438.5 (M), consistent with the calculated value (M 计算值 =1438.6), as attached figure 2 And attached image 3 shown.
[0063] Prepared using the Iodogen method 123 / 131 The labe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com