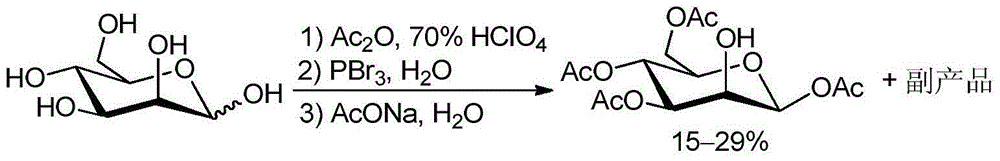
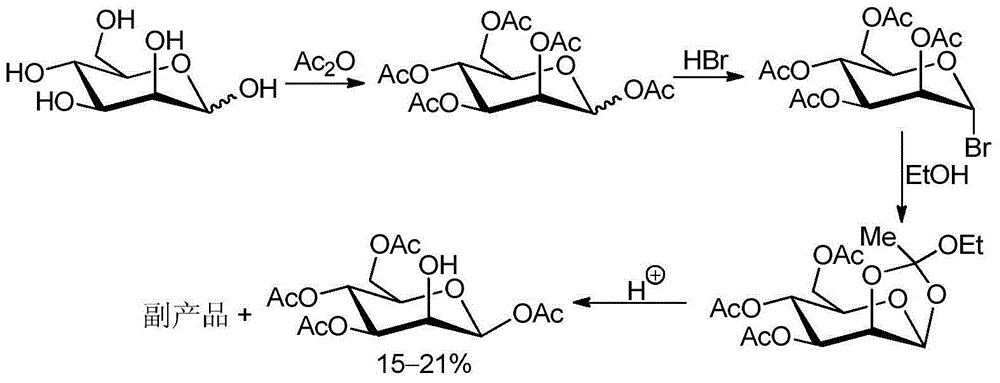
Method for recycling byproduct associated during preparation process of mannose triflate intermediate 1,3,4,6-tetraacetyl-beta-D-mannose
A technology of trifluoromannose and tetraacetyl group, which is applied in the field of by-product conversion and utilization in the preparation process of intermediates, can solve the problems of low utilization rate and many associated by-products, etc., so as to improve yield, solve many reaction steps, and reduce environmental pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method for recycling the by-products associated with trifluoromannose intermediate 1,3,4,6-tetraacetyl-β-D-mannose of the present invention comprises the following steps:
[0037] 174 grams of by-products associated with the multi-step one-pot method were added to 174 milliliters of acetic anhydride solution containing 0.17 grams of perchloric acid and 0.17 grams of tetrabutylammonium iodide, and the reaction temperature was controlled at 35°C while stirring. The addition was complete in 30 minutes, followed by stirring at room temperature for 1 hour. Cool down to -5°C, and add 174 ml of pre-cooled 30% hydrogen bromide glacial acetic acid solution to the mixture. After the addition was complete, the mixture was stirred at room temperature for 8 hours. Add 500 milliliters of dichloromethane to the reaction flask again, separate the layers to obtain the organic phase, wash with pre-cooled saturated brine and sodium bicarbonate solution, dry over anhydrous sodium sulfat...
Embodiment 2
[0039] The recycling method of the trifluoromannose intermediate 1,3,4,6-tetraacetyl-β-D-mannose associated by-products of the present invention comprises the following steps:
[0040] 174 grams of by-products associated with the step-by-step process were added to 522 milliliters of acetic anhydride solution containing 0.87 gram of perchloric acid and 0.87 gram of tetrabutylammonium iodide, stirred and controlled at 40° C. for the reaction temperature. The addition was complete in 1 hour, followed by another 2 hours of stirring at room temperature. The temperature was lowered to 0° C., and 522 ml of precooled 30% hydrogen bromide glacial acetic acid solution was added to the mixture. After the addition was complete, the mixture was stirred at room temperature for 12 hours. Add 500 milliliters of dichloromethane to the reaction flask again, separate the layers to obtain the organic phase, wash with pre-cooled saturated brine and sodium bicarbonate solution, dry over anhydrous ...
Embodiment 3
[0042] A method for recycling the by-products associated with trifluoromannose intermediate 1,3,4,6-tetraacetyl-β-D-mannose of the present invention comprises the following steps:
[0043]174 grams of by-products associated with the multi-step one-pot method were added to 348 milliliters of acetic anhydride solution containing 1.74 grams of perchloric acid and 1.74 grams of tetrabutylammonium iodide, and the reaction temperature was controlled at 38°C with stirring. The addition was complete in 45 minutes, followed by another hour of stirring at room temperature. Cool down to -2°C, and add 348 ml of pre-cooled 30% hydrogen bromide glacial acetic acid solution to the mixture. After the addition was complete, the mixture was stirred at room temperature for 10 hours. Add 500 milliliters of dichloromethane to the reaction flask again, separate the layers to obtain the organic phase, wash with pre-cooled saturated brine and sodium bicarbonate solution, dry over anhydrous sodium su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com