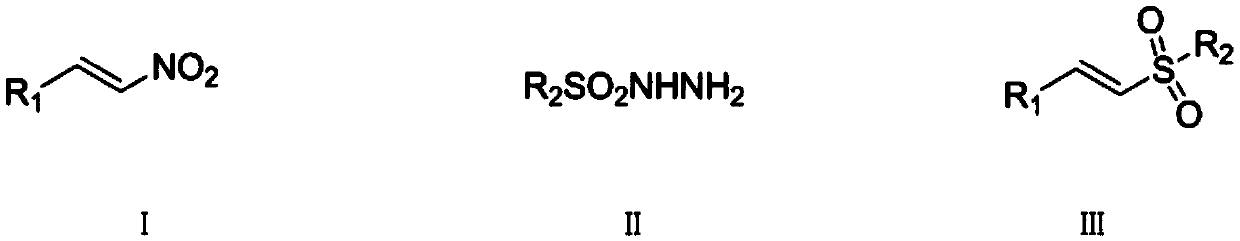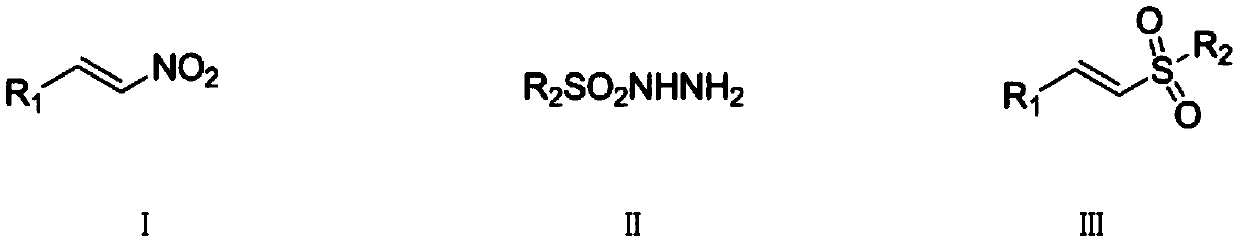Synthetic method of (E)-alkenyl sulfone compounds
A synthetic method and compound technology, applied in the preparation of organic compounds, organic chemical methods, chemical instruments and methods, etc., to achieve the effects of simple synthetic routes, mild reaction conditions, and improved yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] (1) Step 1: Add benzaldehyde (8.8g, 7.8mmol) and nitromethane (4.3g, 7.7mmol) to methanol (15mL), cool to -5°C, stir for half an hour, then slowly add sodium hydroxide solution (15 mL), methanol (15 mL) was added, and the reaction was continued for 1 hour after the dropwise addition was completed. Add crushed ice to the reaction solution, pour it into 4mol / L hydrochloric acid (75mL) after 5min, precipitate a light yellow precipitate, filter, wash with water (30mL×3), transfer the solid to a flask, recrystallize from ethanol, and dry at room temperature 7.9 g of β-nitrostyrene solid was obtained, with a yield of 71%.
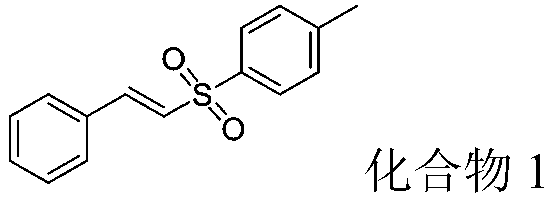
[0043] (2) Step 2: The above-mentioned β-nitrostyrene (74.5mg, 0.5mmol), p-toluenesulfonyl hydrazide (186mg, 1.0mmol), tetrabutylammonium iodide (36.5mg, 0.1mmol) and Potassium persulfate (270.0 mg, 1.0 mmol) was added into the flask, and the mixture was reacted at 80° C. for 8 hours. After the reaction was detected by TLC, it was separated b...
Embodiment 2
[0046]
[0047] (1) Step 1: Mix p-tolualdehyde (3.48g, 29mmol), ammonium acetate (1.3g, 1.7mmol), acetic acid (7.5mL), and nitromethane (2g, 33mmol), heat and reflux for four hours, The reaction solution was cooled to room temperature, poured into ice water (25mL), precipitated, stirred, filtered, washed with water (30mL×3), and the crude product was recrystallized with ethanol to obtain the product β-p-methylnitrostyrene as a solid (3.3 g), 72% yield.
[0048] (2) Step 2: Mix the above β-p-methylnitrostyrene (81.5mg, 0.5mmol), p-toluenesulfonyl hydrazide (93mg, 0.5mmol), tetrabutylammonium iodide (91.3mg, 0.25 mmol) and potassium persulfate (135 mg, 0.5 mmol) were added into the flask, and the mixture was reacted at 80° C. for 8 hours. After TLC detection reaction finishes, use column chromatography (eluent: sherwood oil / ethyl acetate volume ratio 9:1) to separate and obtain 1-(4-methylphenyl)-2-p-toluenesulfonyl olefin ( Compound 2) 85.7 mg, yield 63%.
[0049] Product...
Embodiment 3
[0051]
[0052] (1) Step 1: Mix p-chlorobenzaldehyde (4.06g, 29mmol), ammonium acetate (1.3g, 1.7mmol), acetic acid (7.5mL), and nitromethane (2g, 33mmol), heat and reflux for four hours, and The reaction solution was cooled to room temperature, poured into ice water (25mL), precipitated, stirred, filtered, washed with water (30mL×3), and the crude product was recrystallized with ethanol to obtain the product β-p-chloronitrostyrene solid (4.3 g), yield 80%.
[0053] (2) Step 2: The above-mentioned β-p-chloronitrostyrene (91.5mg, 0.5mmol), p-toluenesulfonyl hydrazide (930mg, 5.0mmol), tetrabutylammonium iodide (182.5mg, 0.5mmol) ) and ammonium persulfate (570 mg, 2.5 mmol) were added into the flask, and the mixture was reacted at 80° C. for 8 hours. After the TLC detection reaction finishes, use column chromatography (eluent: petroleum ether / ethyl acetate volume ratio 9:1) to separate and obtain 1-(4-chlorophenyl)-2-p-toluenesulfonyl alkene (compound 3) 58.4 mg, yield 40%....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com