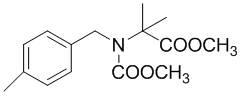
Method for synthesizing 2-(N-4-methyl benzyl) methoxy-acetamido methyl isobutyrate
A technology of methyl methoxyacetamido isobutyrate and methyl benzylidene aminoacetate, applied in the field of synthesis of 2-(N-4-methylbenzyl) methyl methoxyacetamido isobutyrate, Achieve the effect of reducing air pollution, low cost and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] a. Preparation of electrolyte
[0018] Mix 0.001 mol (0.191g) methyl p-methylbenzylidene aminoacetate with 0.001 mol (0.369g) tetra-n-butylammonium iodide and 0.129 mol (10 mL) N,N-dimethylformamide to form an electrolyte , and then placed in a one-chamber electrolytic cell with stainless steel as the cathode and magnesium rod as the anode, methyl p-methylbenzylidene aminoacetate, tetra-n-butylammonium iodide and N,N-dimethylformamide as analytically pure , wherein: methyl p-methylbenzylidene aminoacetate is the substrate, N,N-dimethylformamide is the solvent after drying with 4? grade molecular sieves, and tetra-n-butylammonium iodide is the supporting electrolyte.
[0019] b. Electrocarboxylation reaction
[0020] Under normal pressure, carbon dioxide is introduced into the electrolytic cell to saturation, and then at 2.9mA / cm 2 The constant current electric carboxylation reaction is carried out at a current density of 96.5°C and the reaction temperature is 25°C.
...
Embodiment 2
[0026] a. Preparation of electrolyte
[0027] Mix 0.001 mol (0.191g) methyl p-methylbenzylidene aminoacetate with 0.001 mol (0.369g) tetra-n-butylammonium iodide and 0.129 mol (10 mL) N,N-dimethylformamide to form an electrolyte , and then placed in a one-chamber electrolytic cell with stainless steel as the cathode and magnesium rod as the anode, methyl p-methylbenzylidene aminoacetate, tetra-n-butylammonium iodide and N,N-dimethylformamide as analytically pure , wherein: methyl p-methylbenzylidene aminoacetate is the substrate, N,N-dimethylformamide is the solvent after drying with 4? grade molecular sieves, and tetra-n-butylammonium iodide is the supporting electrolyte.
[0028] b. Electrocarboxylation reaction
[0029] Under normal pressure, carbon dioxide is introduced into the electrolytic cell to saturation, and then at 2.9mA / cm 2 The constant current electric carboxylation reaction is carried out at a current density of 193°C, and the reaction temperature is 25°C.
...
Embodiment 3
[0035] a. Preparation of electrolyte
[0036] Mix 0.001 mol (0.191g) methyl p-methylbenzylidene aminoacetate with 0.001 mol (0.369g) tetra-n-butylammonium iodide and 0.129 mol (10 mL) N,N-dimethylformamide to form an electrolyte , and then placed in a one-chamber electrolytic cell with stainless steel as the cathode and magnesium rod as the anode, methyl p-methylbenzylidene aminoacetate, tetra-n-butylammonium iodide and N,N-dimethylformamide as analytically pure , wherein: methyl p-methylbenzylidene aminoacetate is the substrate, N,N-dimethylformamide is the solvent after drying with 4? grade molecular sieves, and tetra-n-butylammonium iodide is the supporting electrolyte.
[0037] b. Electrocarboxylation reaction
[0038] Under normal pressure, carbon dioxide is passed into the electrolytic cell to saturation, and then at 3.5mA / cm 2 The constant current electric carboxylation reaction is carried out at a current density of 386°C, and the reaction temperature is 22°C.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com