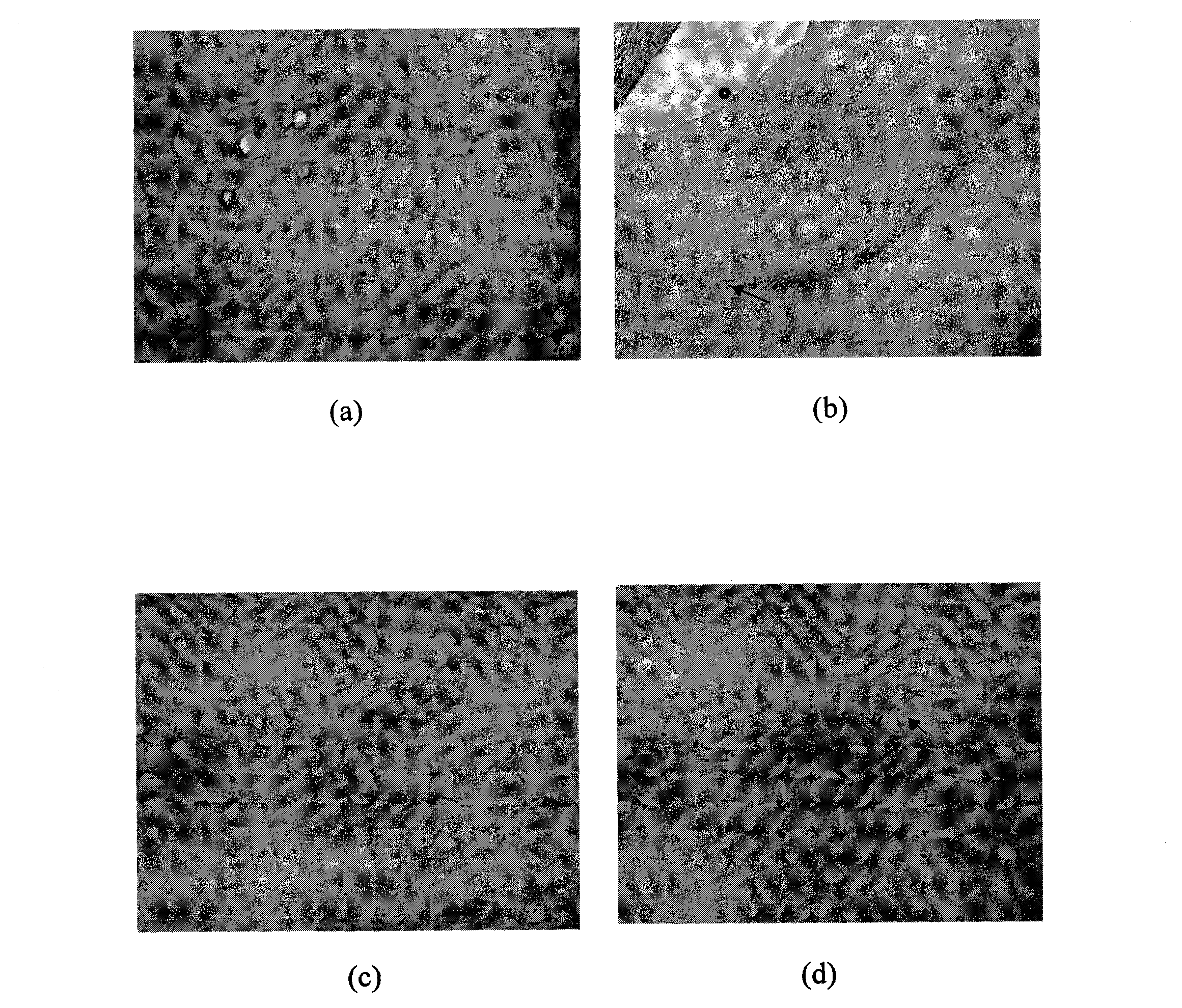
Novel application of 2,4-dimethoxyl trans-stilben
A kind of technology of dimethoxy trans-stilbene and application, applied in the field of lipid-lowering and anti-senile dementia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] 2, the preparation of 4-dimethoxy trans stilbene (hereinafter referred to as S3)
[0050]
[0051] Compound 1 (benzylated bromide, 0.1mol) was reacted with slightly excess triethyl phosphite (0.125mol), the oil bath was heated to 120°C, and the reaction took about 3 hours until no gas escaped, and compound 2 (light yellow oily liquid).
[0052] Put the compound 2 (0.02mol) prepared in the previous step reaction in a 100ml round-bottom flask, place the reaction flask in an ice-water bath, add anhydrous tetrahydrofuran, add NaH (0.02mol) under stirring, react for 2 hours, add compound 3 (0.0126mol), after stirring for 2 hours, stir overnight at room temperature.
[0053] The reaction solution was filtered, the filtrate was concentrated until no THF was evaporated, and the solid was recrystallized from a solution of water: methanol at a ratio of 2:1 to obtain white needle crystals, which were compound 4, namely 2,4-dimethoxystilbene (S3 ), this step yield is 80.1%.
...
Embodiment 2
[0057] S3 vs Aβ 25-35 Protective Effect of Intracerebroventricular Injection Induced Memory Deficiency in Mice
[0058] (1) Experimental method
[0059] 1. Experimental animals Balb / c mice, female, 6 weeks old, were purchased from the Institute of Zoology, Chinese Academy of Medical Sciences.
[0060] 2. Drug dosage and grouping Divided into 4 groups: control group, model group, S3 high-dose group (50 mg / kg / day) obtained by using Example 1 of the present invention, S3 low-dose group (25 mg / kg / day). There were 15 animals in each group. Administration method: use 0.5wt% sodium carboxymethyl cellulose as a solvent to prepare the drug suspension of S3, and start intragastric administration from the day of operation, 0.2ml / monkey / day. The control group and the model group were gavaged with the same amount of vehicle.
[0061] 3. Aβ 25-35 Intraventricular injection Animals were kept in a clean animal room of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sc...
Embodiment 3
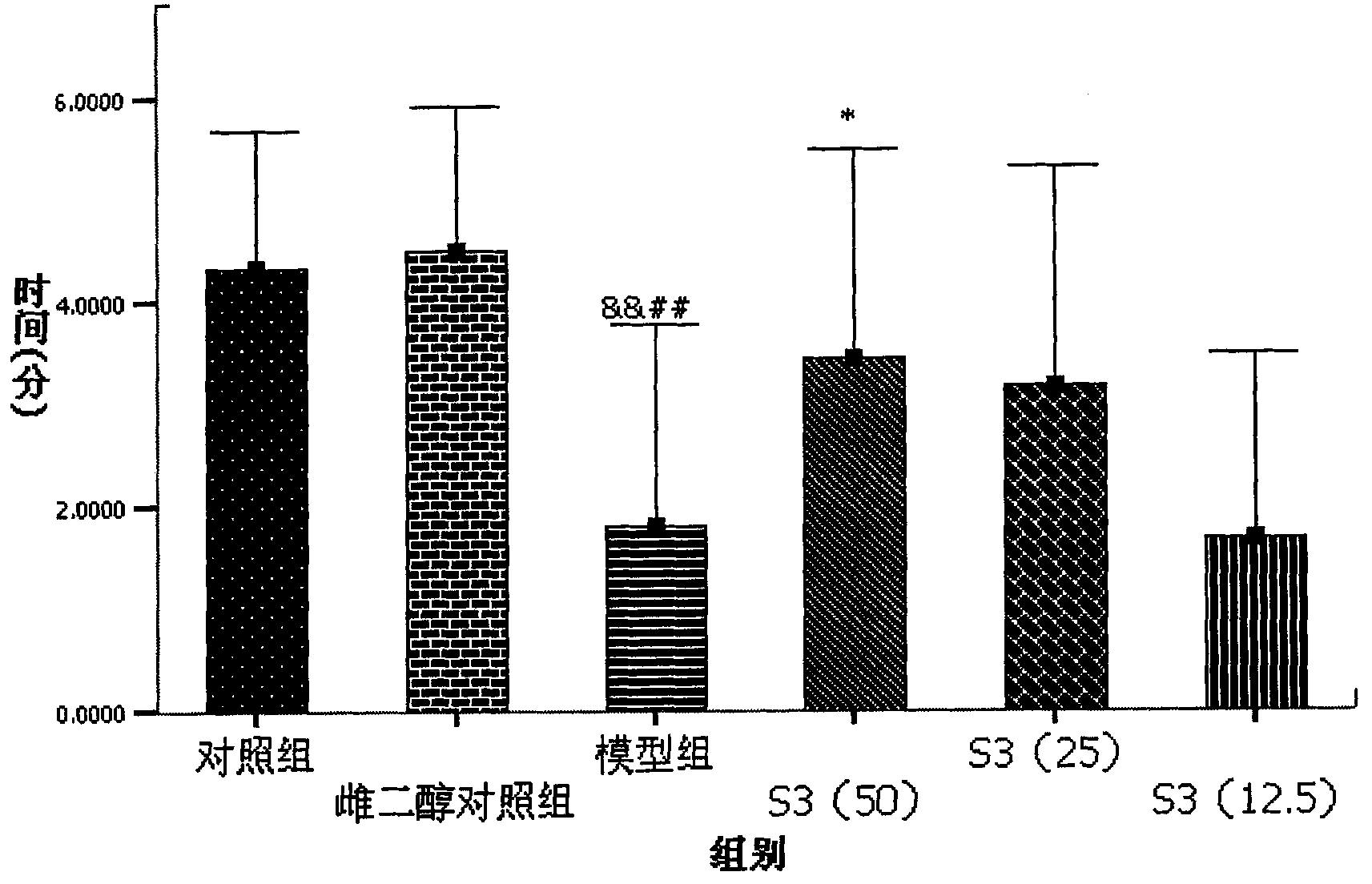
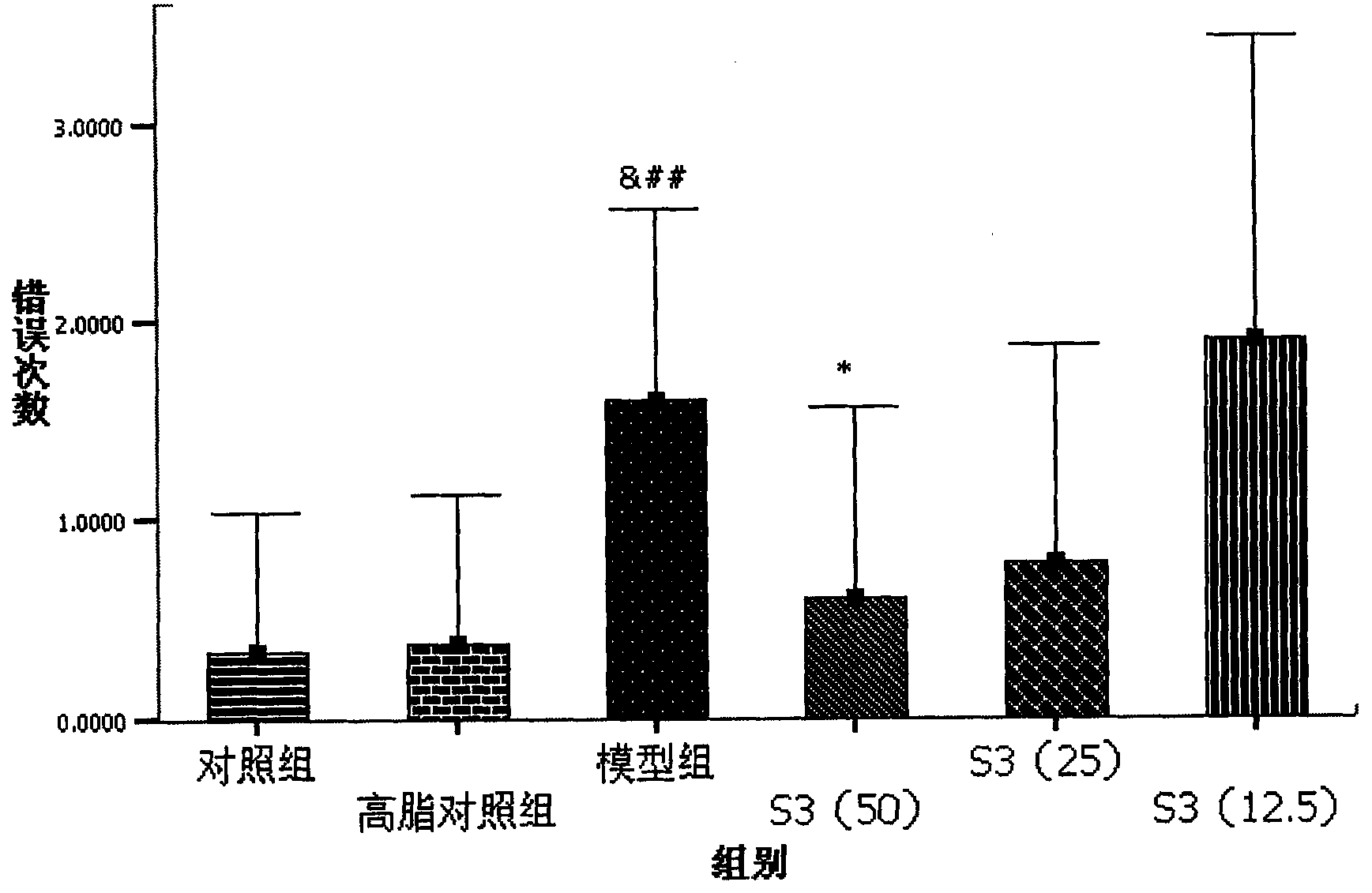
[0091] S3 to high fat+Aβ 25-35 Protective Effect of Intracerebroventricular Injection Induced Memory Deficiency in Rats
[0092] (1) Experimental method
[0093] 1. Experimental animals: wistar rats, female, 180 g, purchased from the Institute of Zoology, Chinese Academy of Medical Sciences, license number: SCXK (Beijing) 2005-0013.
[0094] 2. Drug dosage and grouping: the animals were divided into 6 groups, which were normal control group, high-fat control group, and model group. The commercially available S3 high-dose group (50mg / kg / day), S3 middle-dose group (25mg / kg / day), S3 low-dose group (12.5mg / kg / day). There were 10 animals in each group.
[0095] 3. Aβ 25-35 Intraventricular injection: Animals were kept in a clean animal room of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, and the feed and drinking water were sterilized. After all the animals were fed with ordinary feed for one week, all the animals were fed with high-fat feed f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com