Method and device for preparing high-purity boron tribromide
A boron tribromide, high-purity technology, applied in the direction of boron halide compounds, etc., can solve the problems of low purity of synthetic products, affecting the performance of high-purity boron bromide, etc., and achieve the effect of less impurities, simple equipment and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
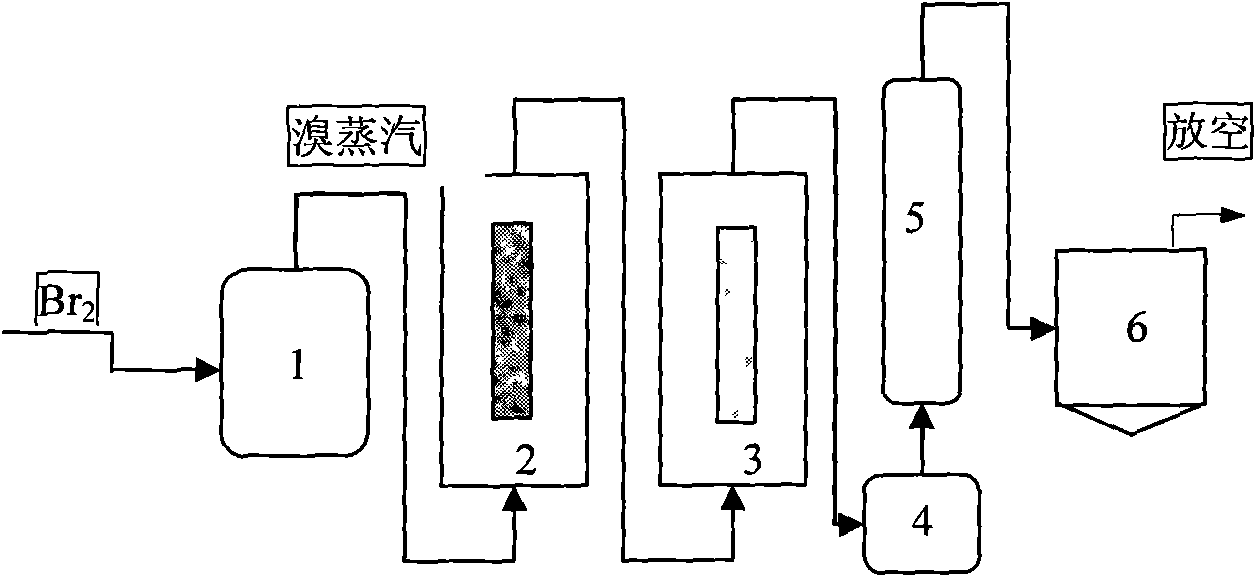
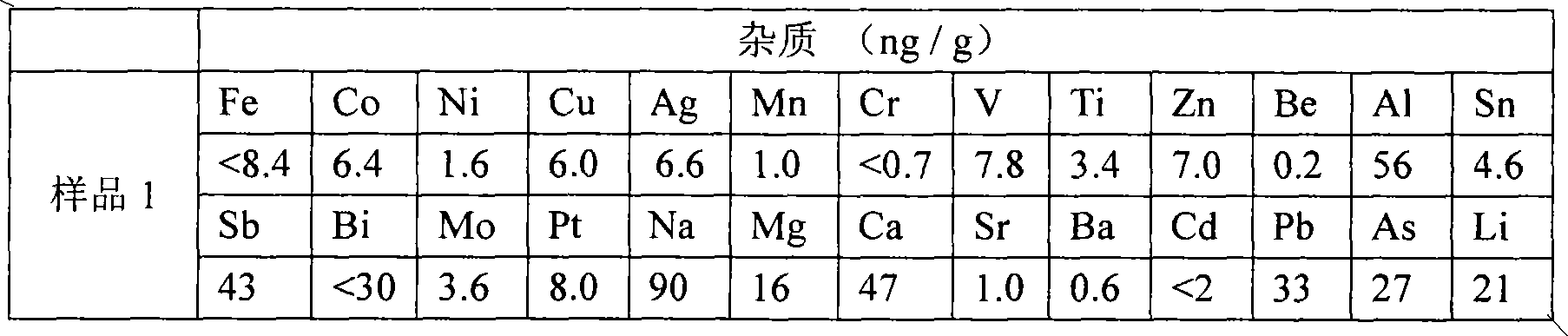
[0036]500g of industrial boron powder (80%-98%) was washed with 5L0.3mol / L hydrochloric acid to make a boron block and put it into a bromination furnace. After the temperature rose to 600°C, 5kg of liquid bromine (> 99.5%) enters the bromination furnace 2 through the pipeline from the bottom after being vaporized by the vaporizer 1, and the gasification temperature is 45° C. to obtain red fuming gas. , react with the 5N high-purity aluminum sheet in the furnace at a temperature of 50°C to obtain white boron bromide gas, the resulting gas is water-cooled to form a white liquid that flows into the collector 4, and the temperature of the collector 4 is controlled so that boron bromide passes through the outlet of the collector. The pipeline enters the fractionation column 5 for fractionation, and the low-boiling point impurities evaporate before boron bromide, while the high-boiling point impurities remain in the collector 4, and the fraction at 102-105°C is selected, which is the...
Embodiment 2
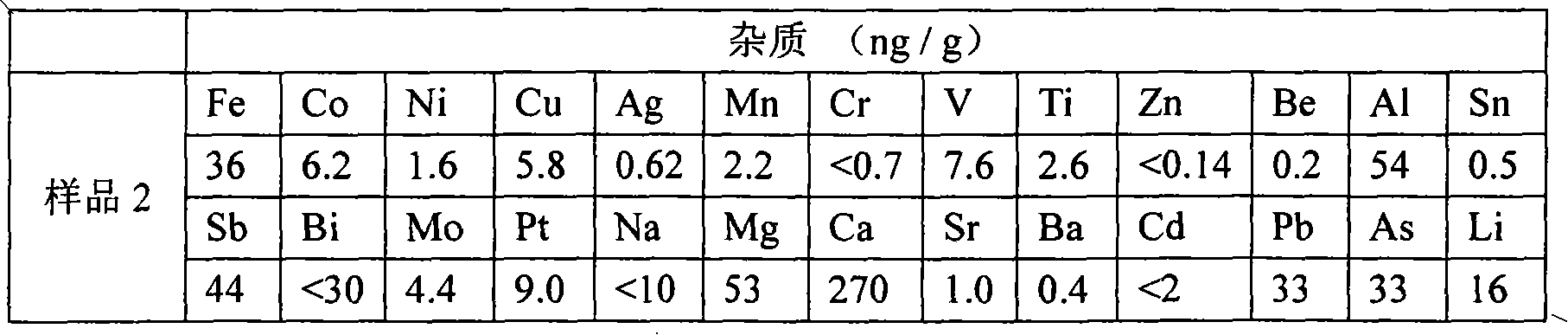
[0040] 500g industrial boron powder (80%-98%) is made into boron block after being washed with 3L1mol / L sulfuric acid and is packed in the bromination furnace 2. After the temperature rises to 850°C, 5kg liquid bromine (>99.5 %) enters the bromination furnace 2 through the pipeline from the bottom after being gasified by the vaporizer 1, and the gasification temperature is 50° C. to obtain red fuming gas. React with 4N high-purity aluminum flakes in the furnace at a temperature of 150°C to obtain white boron bromide gas. The resulting gas is water-cooled to form a white liquid and flows into collector 4. Control the temperature of collector 4 so that boron bromide passes through the pipe from the outlet of the collector. After entering the fractionation column 5 for fractionation, the low-boiling point impurities evaporate before the boron bromide, and the high-boiling point impurities remain in the collector 4, and the fraction at 95-98°C is selected, which is the evaporation ...
Embodiment 3
[0044] 500g industrial boron powder (80%-98%) is made into boron block after being washed with 2L2mol / L nitric acid and is packed in the bromination furnace 2. After the temperature rises to 770° C., 5 kg of liquid bromine (>99.5 %) enters the bromination furnace 2 through the pipeline from the bottom after being gasified by the vaporizer 1, and the gasification temperature is 60° C. to obtain red fuming gas. React with 6N high-purity aluminum flakes in the furnace at a temperature of 100°C to obtain white boron bromide gas. The resulting gas is water-cooled to form a white liquid and flows into collector 4. Control the temperature of collector 4 so that boron bromide passes through the pipeline from the outlet of the collector After entering the fractionation column 5 for fractionation, the low-boiling point impurities evaporate before the boron bromide, and the high-boiling point impurities remain in the collector 4, and the fraction at 117-120°C is selected, which is the eva...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com