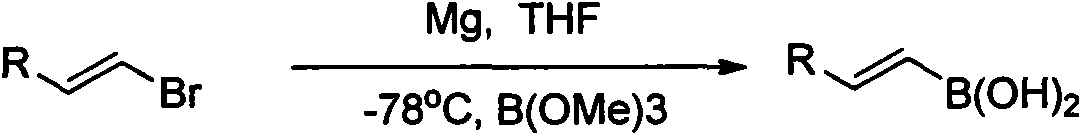
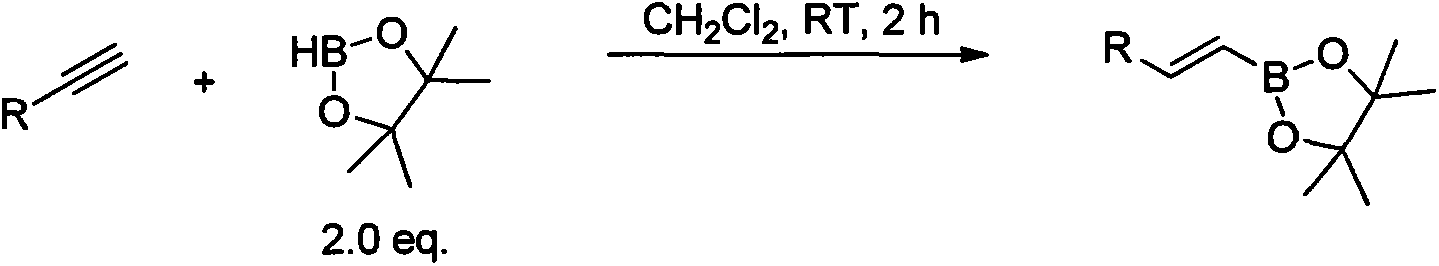Synthesis and application of novel vinyl boronizing reagent
A technology of vinyl boron and reagents, applied in the field of boron chemical synthesis, can solve the problems of high price and high price of metal ruthenium reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of trans-bromovinyl potassium trifluoroborate (1): under the protection of argon, in a 5L three-neck flask equipped with ventilation and airtight buffer system, first add boron tribromide (250 grams, 1.0 mole) and anhydrous 1500 ml of dichloromethane, stirred evenly, cooled to -10°C. Start to feed acetylene gas slowly, and keep the gas bubbling from below the liquid surface during the feeding. At this time, there will be obvious heat generation, and keep the system temperature not exceeding 0°C and continue feeding. The entire aeration process is completed within 4-8 hours. If the aeration is too fast, the cooling should be accelerated or the aeration should be stopped to avoid excessive pressure in the system. When there is no longer gas absorption, about 1.4 moles (36.5 grams) of acetylene are passed into. At this time, the reaction solution is carefully sampled. The reaction solution is added to the previously cooled pinacol and methanol mixed solution, and...
Embodiment 2
[0033] Synthesis of trans-bromovinyl potassium trifluoroborate (1): under the protection of argon, in a 10L glass kettle equipped with ventilation and a closed buffer system, first add boron tribromide (500 grams, 2.0 moles) and anhydrous 2500 ml of dichloromethane, after stirring evenly, cooled to -10°C. Start to feed acetylene gas slowly, and keep the gas bubbling from under the liquid surface when feeding. At this time, there will be obvious heat release, and keep the system temperature not exceeding 0°C and continue feeding. The entire aeration process is completed within 6-10 hours. If the aeration is too fast, the cooling should be accelerated or the aeration should be stopped to avoid excessive pressure in the system. When there is no longer gas absorption, about 3.0 moles (78.1 grams) of acetylene are passed into, and the reaction solution is carefully sampled at this time, and the reaction solution is added to the previously cooled pinacol and methanol mixed solution,...
Embodiment 3
[0036]Synthesis of trans-1-propenyl potassium trifluoroborate (3, R=Me): In a fully dried 250 ml three-necked flask, 0.05 mole of boronating reagent 1 (10.6 g), methyl boric acid (0.06 mole, 3.6 grams) and 150 milliliters of toluene were added, and after stirring evenly, the trachea was inserted until the solution was below the liquid level, and nitrogen was bubbled to remove oxygen for about 10-20 minutes. Subsequently, anhydrous potassium fluoride (0.125 mol, 7.26 g) and PdCl2 (dppf) (1.1 g, 3% mol) were added under nitrogen protection, and reacted at 45° C. overnight. After the detection reaction is finished, filter with diatomaceous earth and wash with 30 ml of toluene. After the combined filtrates were spin-dried under reduced pressure, 160 ml of acetone was added, the insoluble solids were filtered off, the filtrate was spin-dried again, 110 ml of diethyl ether was added for beating, and 4.59 g of off-white flaky solids were obtained with a yield of 62%. H NMR (400MHz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com