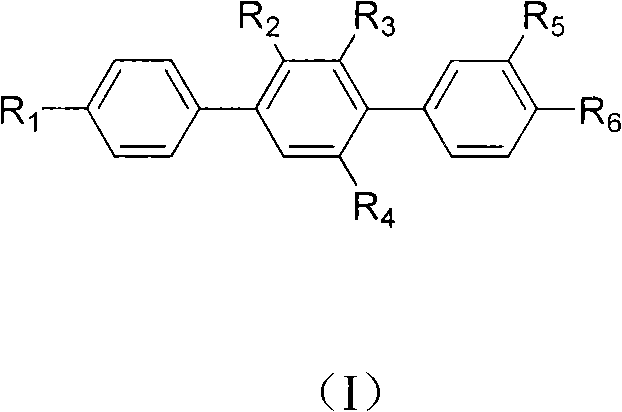
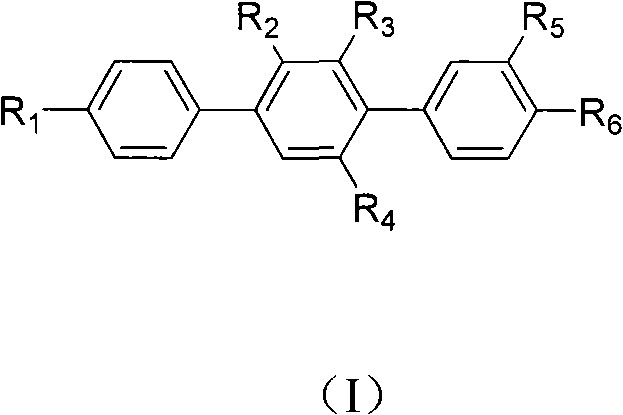
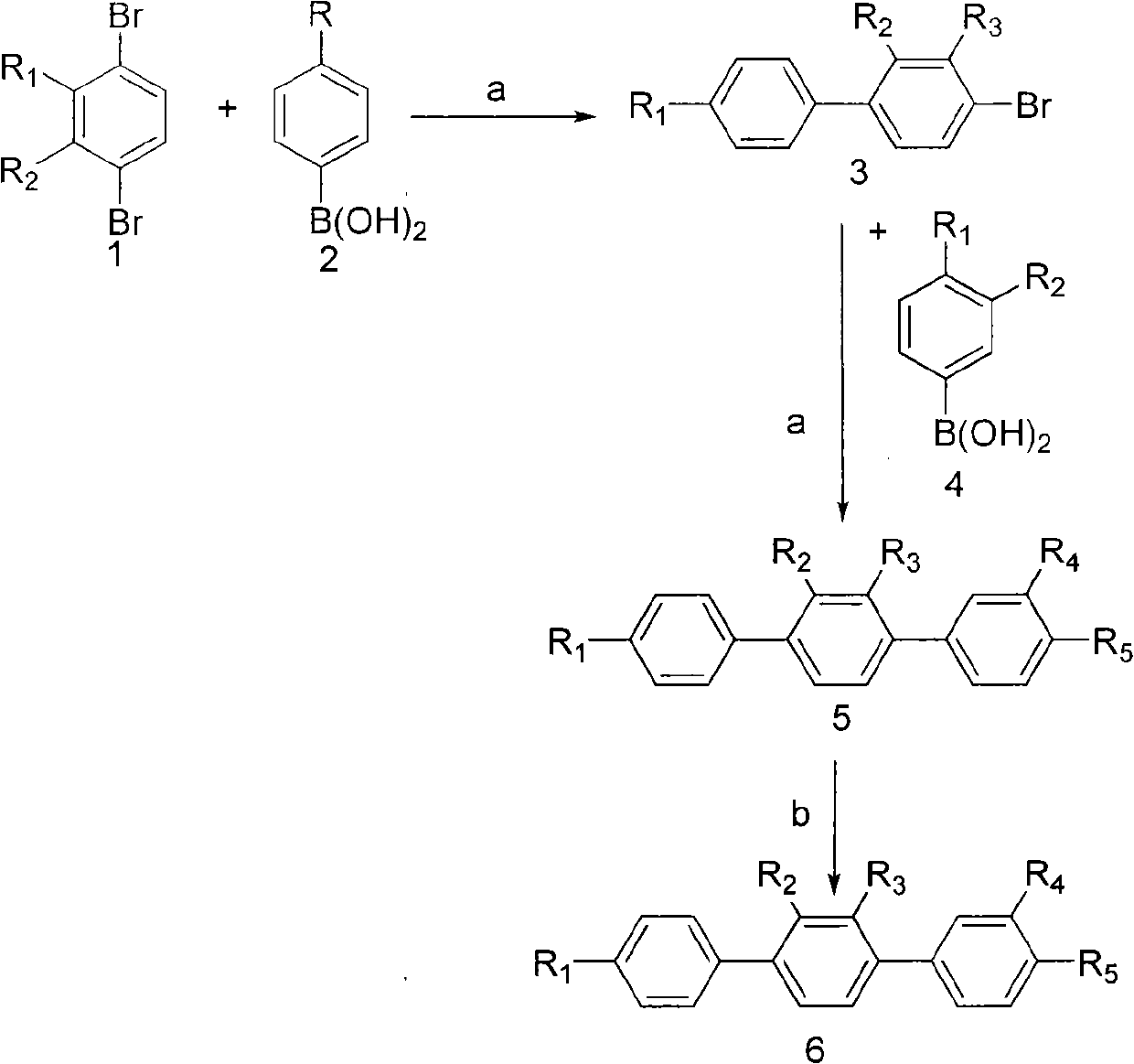
Paraterphenyl derivative and application thereof to preparation of antitumor medicaments
An anti-tumor drug, p-terphenyl technology, applied in the direction of anti-tumor drugs, organic compound preparation, drug combination, etc., can solve the problems of terphenyl complex structure, complicated synthesis, high cost, etc., and achieve low cost and high cytotoxic activity , Synthetic convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Prepare compound [1,1'-Biphenyl]-4'-monol, 4-bromo-2,3-dimethoxy-(structural formula 3, wherein R 1 =OH, R 2 = R 3 =OCH 3 ,)
[0037] Put p-hydroxyphenylboronic acid (1 mole) and 1,4-dibromo-2,3-dimethoxy-benzene (2 moles) into dioxane (10 ml), add KF (3 moles), nitrogen Pd-DPPF (2% mol) was added under protection, heated to reflux for 24 hours, extracted with ethyl acetate, and the extract was purified by silica gel column chromatography, eluting with petroleum ether: ethyl acetate = 10:1. Mp117~118℃.
[0038] 1 H NMR (300MHz, DMSO-d6, rt) δ3.57(s, 3H), 3.58(s, 3H), 6.83(d, J=15.0Hz, 2H), 7.01(d, J=15.0Hz, 1H) , 7.32(s, J=15.0Hz, 2H), 7.37(d, J=14.0Hz, 1H), 9.58(s, 1H); 13 C NMR (75MHz, CDCl 3 , rt) δ60.8, 60.9, 115.2, 115.2, 116.2, 126.4, 127.9, 129.7, 130.3, 130.3, 135.6, 150.7, 151.5, 155.1; HRMS (ESI) calcd for C 14 h 13 BrNaO 3 (M+H) +330.9946, found 330.9947.
Embodiment 2
[0040] Prepare compound [1,1':4',1 "-Terphenyl]-4"-monol, 3,4-dimethoxy-(9CI) (structural formula 5, wherein R 1 =OH,R 2 =R 3 = H, R 4 =R 5 =OCH 3 )
[0041] Put 3,4 dimethoxyphenylboronic acid (1.5 moles) and 1,1'-biphenyl-4-hydroxyl, 4'-bromo (1 mole) into dioxane (10ml), add KF (3 mol), Pd-DPPF (2% mol) was added under nitrogen protection, heated to reflux for 24 hours, extracted with ethyl acetate, and the extract was purified by silica gel column chromatography, eluting with petroleum ether:ethyl acetate=10:1. Mp250~252℃.
[0042] 1 H NMR (300MHz, DMSO-d6, rt) δ3.77(s, 3H), 3.92(s, 3H), 6.87(d, J=14.0Hz, 2H), 7.04(d, J=14.0Hz, 1H) , 7.23(d, J=14.0Hz, 1H), 7.26(s, 1H), 7.54(d, J=14.0Hz, 2H), 7.64(d, J=14.0Hz, 2H), 7.70(d, J= 13.0Hz, 2H), 9.59(s, 1H); 13 C NMR (75MHz, CDCl 3 , rt) δ109.99, 112.0, 115.6, 115.6, 118.4, 126.1, 126.1, 126.6, 126.6, 127.5, 127.5, 130.3, 132.4, 137.9, 138.4, 148.3, 148.9, 157.0; HRMS (ESI) calcdforC 20 h 19 o 3 (M+H) + 307.1334, fo...
Embodiment 3
[0044] Prepare compound [1,1':4',1 "-Terphenyl]-3,4,4"-triol (9CI) (structural formula 6, wherein R 1 =OH,R 2 =R 3 = H, R 4 =R 5 =OH)
[0045] Dissolve [1,1':4',1"-Terphenyl]-4"-monol, 3,4-dimethoxy-(9CI) (1 mole) in dichloromethane, lower the temperature to -45°C, add three Boron bromide (1.5 mol), warmed up, stirred for 24 hours, extracted with ethyl acetate, and the extract was purified by silica gel column chromatography, eluting with petroleum ether:ethyl acetate=10:3 to obtain the compound. Mp>260°C.
[0046] 1 H NMR (300MHz, DMSO-d6, rt) δ6.82 (d, J = 10.0Hz, 1H), 6.85 (d, J = 10.0Hz, 2H), 6.97 (d, J = 10.0Hz, 1H), 7.10 (s, 1H), 7.51(d, J=10.0Hz, 2H), 7.55(d, J=10.0Hz, 2H), 7.60(d, J=10.0Hz, 2H), 9.02(s, 1H), 9.05 (s, 1H), 9.54(s, 1H); 13 C DEPT (75MHz, (CD 3 ) 2 CO, rt) δ113.7, 113.7, 115.7, 115.7, 118.2, 118.2, 126.5, 126.5, 126.6, 127.7, 127.7, 131.9, 132.7, 138.99, 139.1, 144.9, 145.4, 157.1; HRMS (ESI) cal cd for C 18 h 15 o 3 (M+H) + 279.1021, found...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com