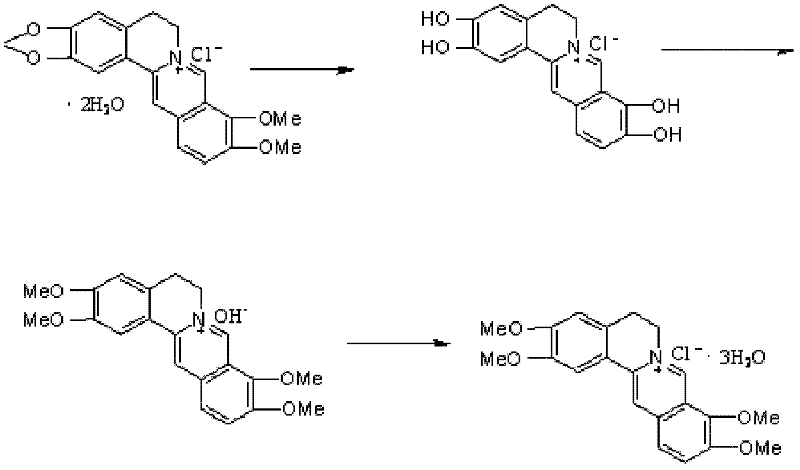
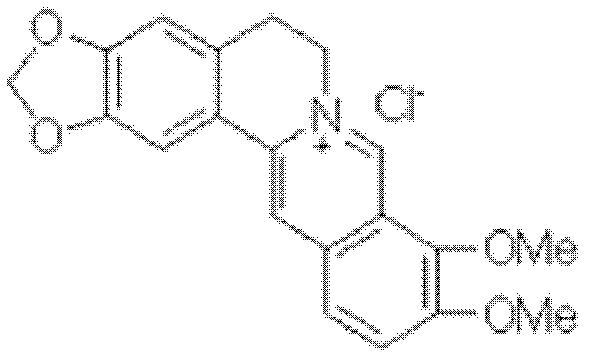
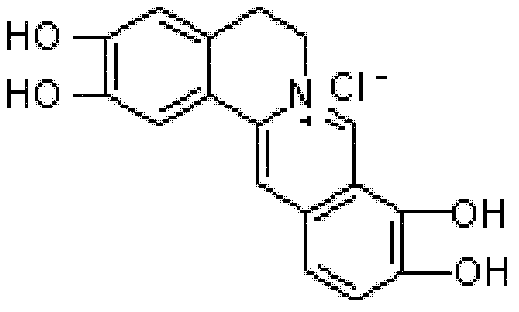
The preparation method of palmatine
A technology of palmetine and organic solvents, which is applied in the field of chemical synthesis of broad-spectrum antibacterial drugs, can solve the problems of complicated operation, time-consuming and labor-consuming, and low content, and achieve the effects of protecting the ecological environment, obtaining cheap and easy raw materials, and saving plant resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Weigh 20g of berberine hydrochloride, 300ml of xylene, and 30g of aluminum trichloride into a 1L reaction bottle equipped with a reflux condenser, thermometer, and tail gas absorption device, and keep the reaction temperature at 140°C for about 4 hours. The reaction is over. Cool down, add 300ml of ice water dropwise, stir thoroughly for 1 hour, filter, wash the filter cake with 100ml of water, drain it, and dry it in a vacuum oven at 40°C for 4 hours to obtain 2,3,9,10-tetrahydroxyberberis hydrochloride Base 18.6g, weight yield 93%.
[0021] (2) Get 9g of 2,3,9,10-tetrahydroxy berberine hydrochloride obtained in the above steps and drop into a 250ml reaction bottle, add 40ml of distilled water and stir, add dimethyl sulfate 12ml, add dropwise 10% NaOH solution 60ml, drop At the end of the addition, the PH value was determined to be alkaline, and the temperature was kept at about 30°C for 2 hours, cooled to 5°C and stirred for 2 hours, filtered, washed with a small...
Embodiment 2
[0024] (1) Weigh 20g of berberine hydrochloride, 400ml of xylene, and 60g of boron tribromide are put into a 1L reaction flask equipped with a reflux condenser, a thermometer, and a tail gas absorption device, and keep the reaction temperature at 100°C for the ring-opening reaction, about The 4 hour reaction was complete. Cool down, add 400ml of ice water dropwise, stir thoroughly for 1 hour, filter, wash the filter cake with 100ml of water, drain it, and dry it in a vacuum oven at 40°C for 4 hours to obtain 2,3,9,10-tetrahydroxyberberis hydrochloride Alkali 19g, weight yield 95%.
[0025] (2) Get 9g of 2,3,9,10-tetrahydroxy berberine hydrochloride obtained in the above steps and drop into a 250ml reaction bottle, add 40ml of distilled water and stir, add 20ml of dimethyl sulfate, add dropwise 100ml of 10% NaOH solution, drop At the end of the addition, the PH value was determined to be alkaline, and the temperature was kept at about 40°C for 4 hours, cooled to 5°C and stirre...
Embodiment 3
[0028] (1) Weigh 20 g of berberine hydrochloride, 300 ml of toluene, and 30 g of aluminum trichloride are put into a 1L reaction bottle equipped with a reflux condenser, a thermometer, and a tail gas absorption device, and keep the reaction temperature at 110° C. for ring-opening reaction, about 4 The hour reaction is over. Cool down, add 300ml of ice water dropwise, stir thoroughly for 1 hour, filter, wash the filter cake with 100ml of water, drain it, and dry it in a vacuum oven at 40°C for 4 hours to obtain 2,3,9,10-tetrahydroxyberberis hydrochloride Base 18.8g, weight yield 94%.
[0029] (2) Get 9g of 2,3,9,10-tetrahydroxy berberine hydrochloride obtained in the above steps and drop into a 250ml reaction bottle, add 30ml of distilled water and stir, add 18ml of dimethyl sulfate, add dropwise 90ml of 10% NaOH solution, drop At the end of the addition, the PH value was determined to be alkaline, and the temperature was kept at about 35°C for 4 hours, cooled to 5°C and stirr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com