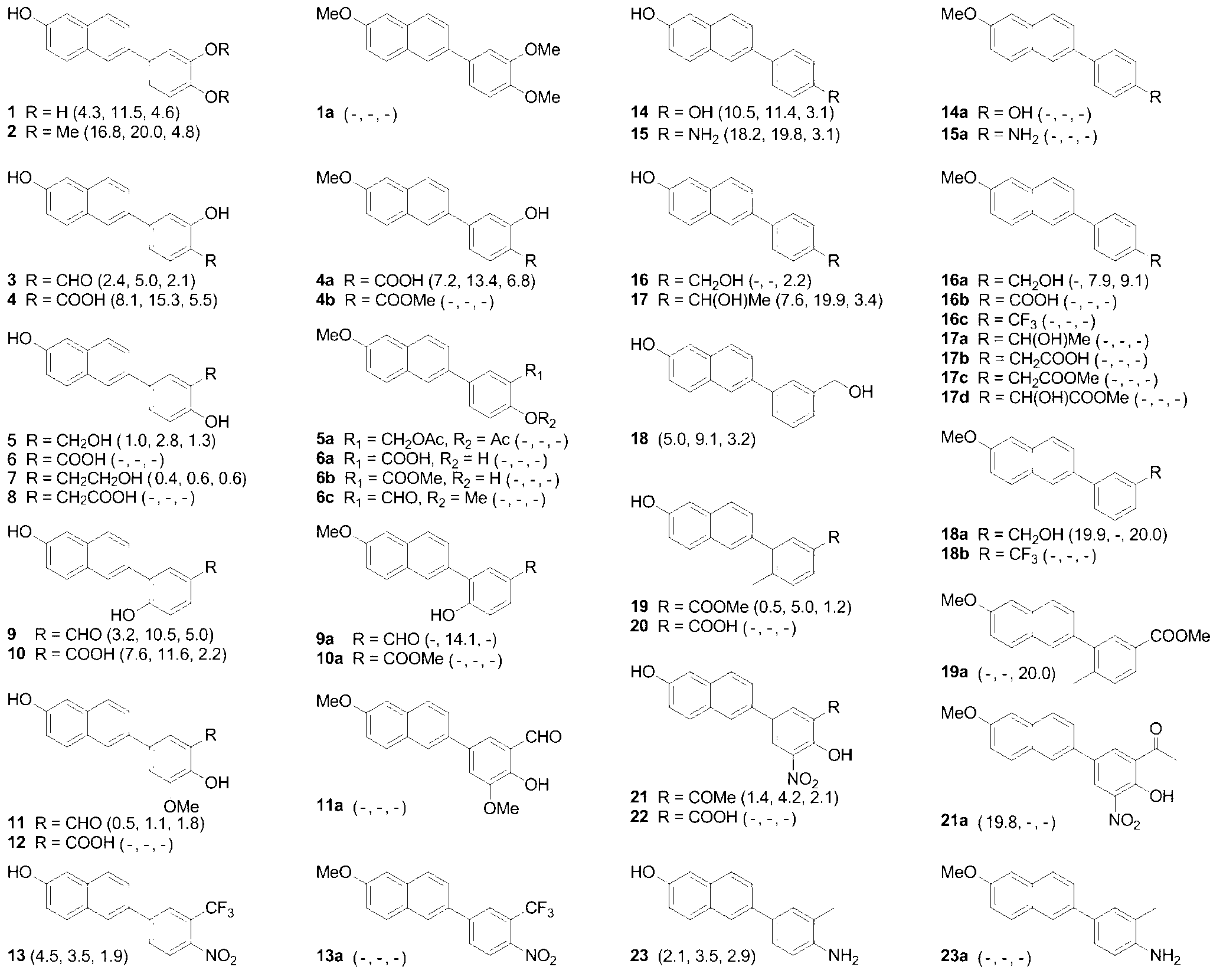
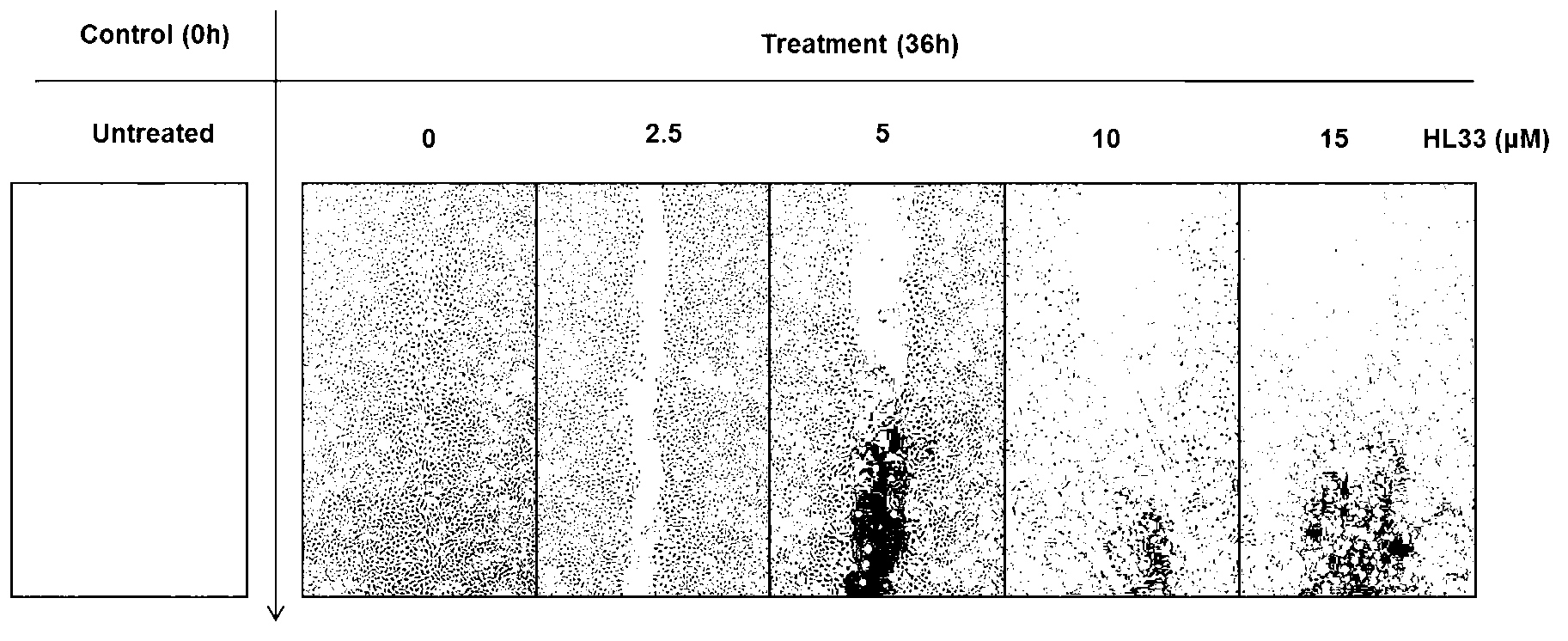
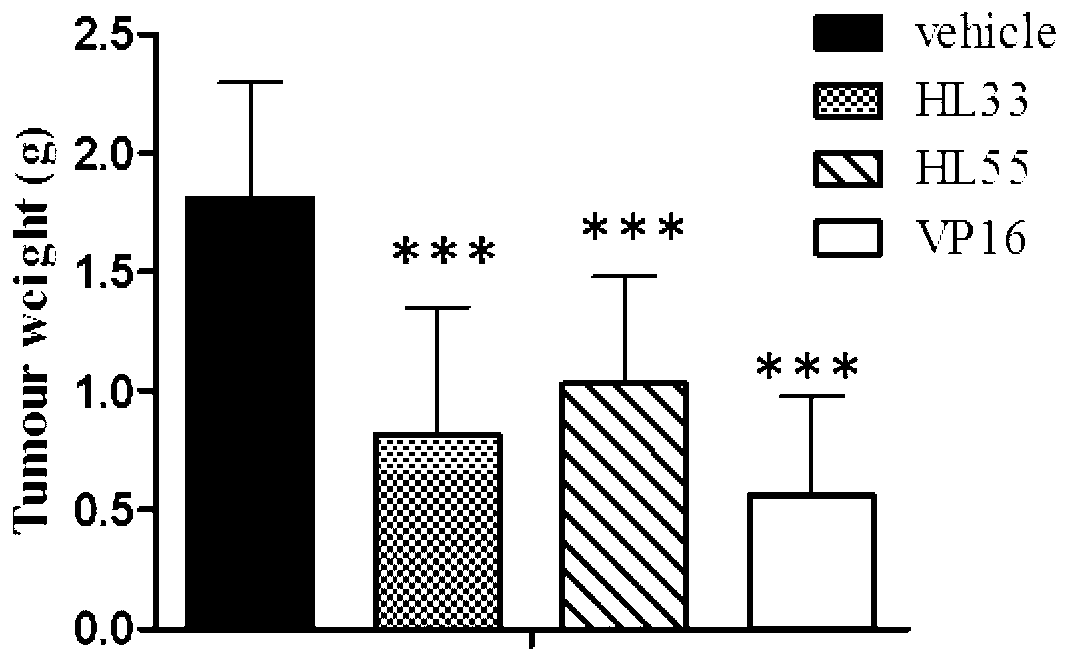
2-phenylnaphthalene derivative and application thereof in preparation of anti-tumor medicaments
An anti-tumor drug, the technology of phenylnaphthalene, which is applied in the field of 2-phenylnaphthalene derivatives and its application in the preparation of anti-tumor drugs, can solve the problems of high cost, complex structure, cumbersome synthesis, etc., and achieve convenient synthesis, The effect of simple structure and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of compound 2-(6-methoxynaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (structural formula 2, wherein R 2 ’=OCH 3 , R 1 ’=R 4 ’ = H)
[0037] Put 2-methoxy-6-naphthol (1 mol) and dipivaloyl diboron (1.2 mol) into dioxane (10ml), add potassium acetate (3 mol), and add Pd- DPPF (2% mol), heated to reflux for 24 hours, extracted with ethyl acetate,
[0038] The extract was purified by silica gel column chromatography, eluting with petroleum ether: acetone = 10:1.
[0039] 1 H NMR (600MHz, CDCl 3 ,δ):8.29(s,1H),7.81(d,J=12.0Hz,1H),7.78(d,J=12.0Hz,1H),7.73(d,J=12.0Hz,1H),7.14(d ,J=12.0Hz,1H),7.12(s,1H),3.93(s,3H),1.39(s,12H); 13 C NMR (125MHz, CDCl 3 ,r.t.):δ158.5,136.41,136.0,131.1,130.3,128.4,125.9,118.7,105.6,83.3,55.3,24.9; MS(ESI):m / z285.2[M+H] + .
[0040] Prepare compound 2-(3,4-dimethoxyphenyl)-6-methoxynaphthalene- (structural formula 4, wherein R 2 ’=R 2 ''=R 3 ''=OCH 3 , R 1 ’=R 4 ’=R 1 ''=R 4 ''=R 5 ''=H)
[0041] Put 3,4-...
Embodiment 2
[0047] Prepare compound 2-methoxy-5-(6-methoxynaphthalen-2-yl)benzaldehyde (structural formula 5, wherein R 2 ’=CHO,R 2 ''=R 3 ''=OCH 3 , R 1 ’=R 4 ’=R 1 ''=R 4 ''=R 5 ''=H)
[0048] 2-Methoxy-5-bromobenzaldehyde (1.5 mol) and 2-(6-methoxynaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (1 mol) Put it in dioxane (10ml), add potassium fluoride (3 mol), add Pd-DPPF (2% mol) under nitrogen protection, heat and reflux for 24 hours, extract with ethyl acetate, and use silica gel column chromatography for the extract Purification, petroleum ether: acetone = 10:1 elution.
[0049] 1 H NMR (600MHz, CDCl 3 ,δ):10.55(s,1H),8.19(d,J=6.0Hz,1H),7.96(s,1H),7.91(dd,J=12.0Hz,J=6.0Hz,1H),7.81(d ,J=6.0Hz,1H),7.79(d,J=6.0Hz,1H),7.69(dd,J=12.0Hz,J=6.0Hz,1H),7.18(dd,J=12.0Hz,J=6.0 Hz,1H),7.16(d,J=6.0Hz,1H),7.11(d,J=6.0Hz,1H),4,04(s,3H),3.94(s,3H); 13 C NMR (100MHz, CDCl 3 ,δ):189.9,161.2,157.8,134.6,134.3,133.8,133.7,129.6,129.2,127.4,126.8,125.5,125.1,125.0,119.3,112.2,105....
Embodiment 3
[0054] Preparation of compound 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)naphthalene-2-ol (structural formula 2, wherein R 2 ’=OH,R 1 ’=R 4 ’ = H)
[0055] Put 2-hydroxy-6-naphthol (1 mole) and dipivaloyl diboron (1.2 moles) into dioxane (10 ml), add potassium acetate (3 moles), and add Pd-DPPF under nitrogen protection ( 2% mol), heated to reflux for 24 hours, extracted with ethyl acetate, and the extract was purified by silica gel column chromatography, eluting with petroleum ether: acetone = 10:1.
[0056] 1 H NMR (600MHz, Acetone-d 6 ,δ):8.31(s,1H),7.95(d,J=12.0Hz,1H),7.86(d,J=12.0Hz,1H),7.80(d,J=12.0Hz,1H),7.21(d ,J=12.0Hz,1H),7.16(s,1H),1.40(s,12H); 13 C NMR (125MHz, Acetone-d 6,δ):159.2,137.3,136.2,131.7,130.68,128.9,126.2,119.1,106.0,83.7,24.7; MS(ESI):m / z271.2[M+H] + .
[0057] Preparation compound 6-(4-hydroxy-3-(hydroxymethyl)phenyl)naphthalen-2-ol (structural formula 5, wherein R 2 ’=CH 2 OH,R 2 ''=R 3 ''=OH,R 1 ’=R 4 ’=R 1 ''=R 4 ''=R 5 ''=H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com