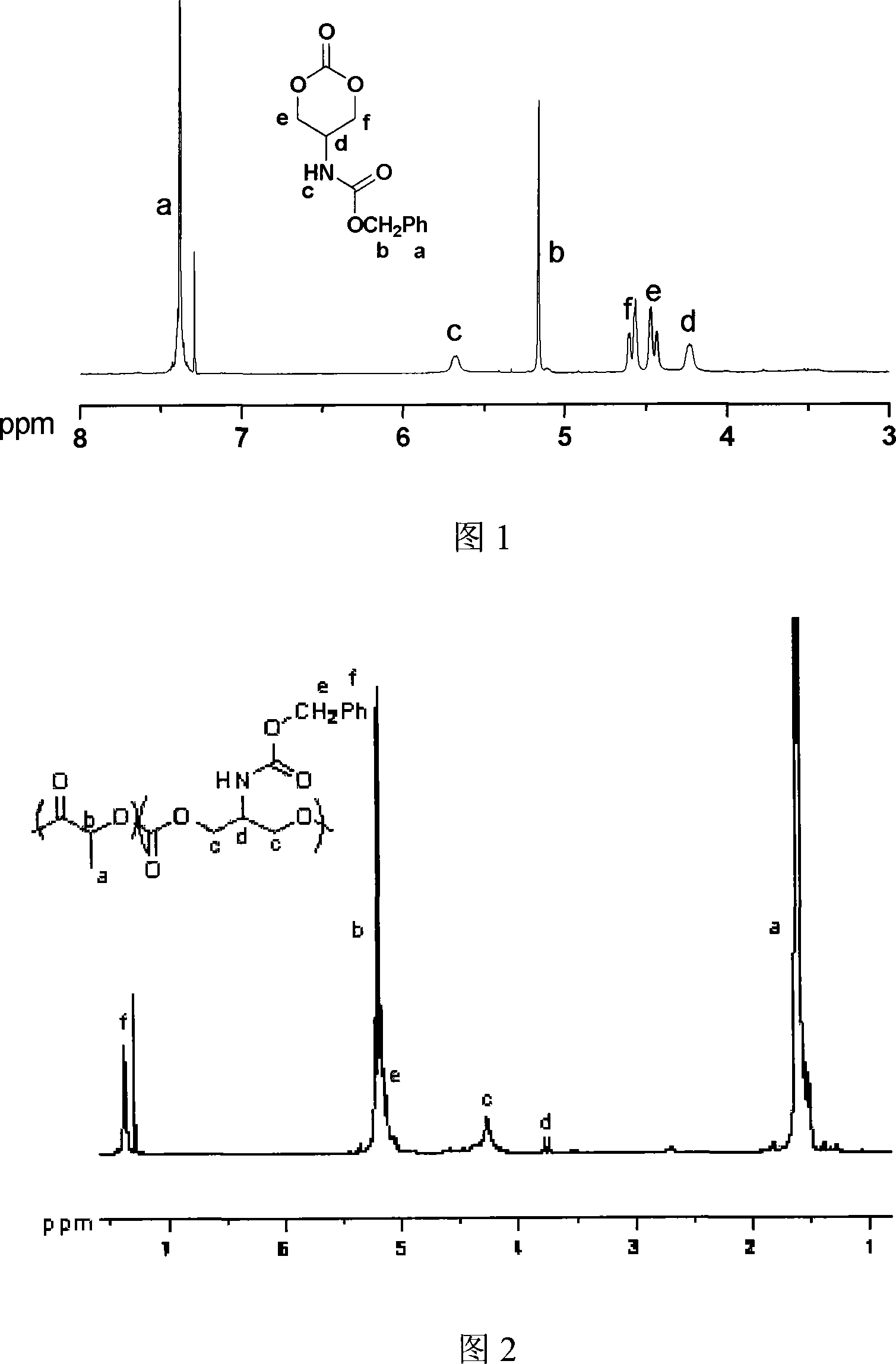
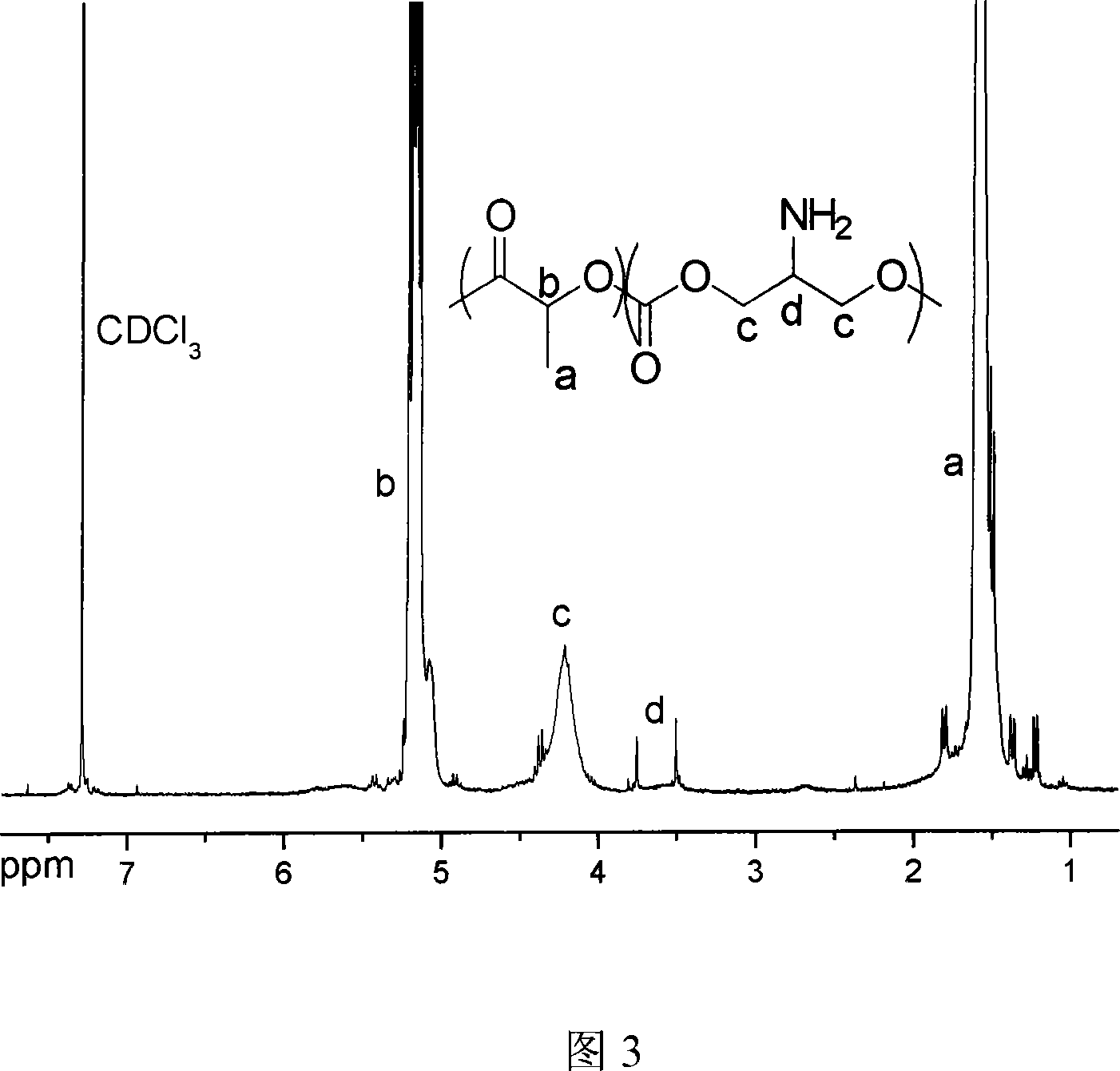
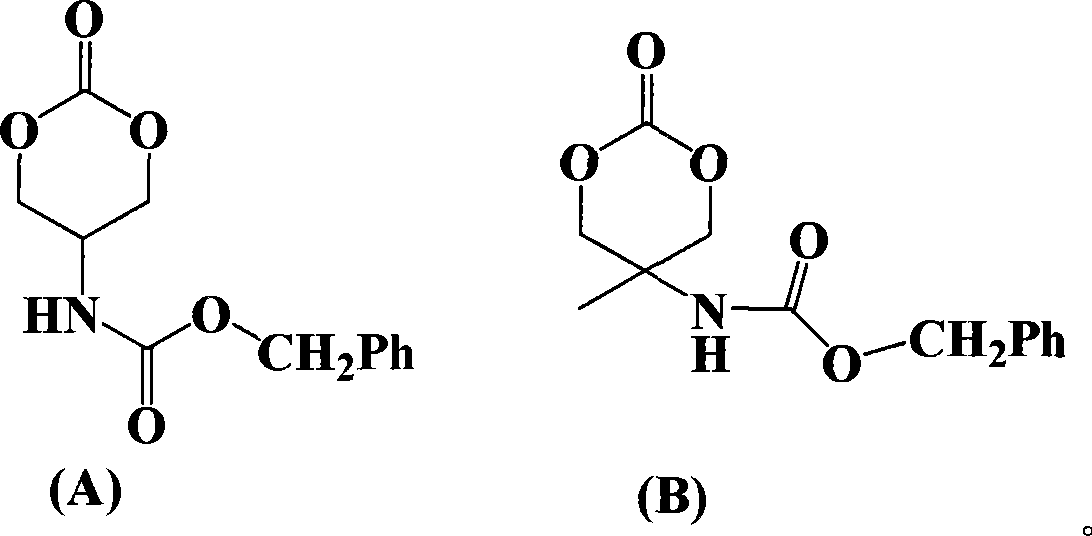
Ring aliphatic carbonate monomer, polymer and preparation method thereof
A technology of family carbonates and cyclic fats, applied in the field of cyclic aliphatic carbonate monomers, their polymers and their preparation, can solve the problems of many reaction steps, complex synthetic routes, and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthesis of 2-benzyloxyamido-1,3-propanediol
[0028]Take 10g (0.11mol) of 2-amino-1,3-propanediol and dissolve it in 250ml of 10% sodium carbonate aqueous solution, then add 125ml of 1,4-dioxane, stir and mix well, place in an ice-water bath Under certain conditions, slowly add 15.7ml (0.11mol) of benzyl chloroformate with a constant pressure dropping funnel, react at room temperature for 8 hours, add 50ml of distilled water to dilute, then extract three times with ethyl acetate, and dry the organic layer with anhydrous magnesium sulfate , concentrated by rotary evaporation, and recrystallized from ethyl acetate to obtain 20 g of 2-benzyloxyamido-1,3-propanediol with a yield of 80%.
Embodiment 2
[0029] Embodiment 2: the synthesis of 2-benzyloxyamido-1,3-propanediol
[0030] Take 10g (0.11mol) of 2-amino-1,3-propanediol and dissolve it in 250ml of 10% sodium carbonate aqueous solution, then add 250ml of 1,4-dioxane, stir and mix well, and place in an ice-water bath Under certain conditions, slowly add 31.4ml (0.22mol) of benzyl chloroformate with a constant pressure dropping funnel, react at room temperature for 8 hours, add 50ml of distilled water to dilute, then extract three times with ethyl acetate, and dry the organic layer with anhydrous magnesium sulfate , concentrated by rotary evaporation, and recrystallized from ethyl acetate to obtain 20.8 g of 2-benzyloxyamido-1,3-propanediol with a yield of 84%.
Embodiment 3
[0031] Example 3: Synthesis of 2-methyl-2-benzyloxyamido-1,3-propanediol
[0032] Take 10g (0.095mol) of 2-methyl-2-amino-1,3-propanediol and dissolve it in 250ml of 10% sodium carbonate aqueous solution, then add 200ml of 1,4-dioxane, stir and mix well , under the condition of an ice-water bath, slowly add 21ml (0.14mol) benzyl chloroformate with a constant pressure dropping funnel, after reacting at room temperature for 8 hours, add 50ml of distilled water to dilute, then extract three times with ethyl acetate, and the organic layer is washed with anhydrous After drying over magnesium sulfate and concentrating by rotary evaporation, recrystallized from ethyl acetate to obtain 17.7 g of 2-methyl-2-benzyloxyamido-1,3-propanediol with a yield of 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com