Method for preparing 2-ethylhexyl chloroformate
A technology of ethylhexyl ester and chloroformic acid, which is applied in the purification/separation of carbonate/haloformate, the chemical method of reacting liquid and gaseous medium, and the method of chemically changing substances by using atmospheric pressure, etc. , can solve the problems of phosgene utilization rate of only 64%, long reaction time, high energy consumption of desolventization, etc., and achieve the effect of solving hydrogen chloride transfer, reducing reaction time and increasing reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The present embodiment provides a kind of method for preparing 2-ethylhexyl chloroformate, described method adopts the reaction kettle of two stages in series, specifically comprises the following steps:
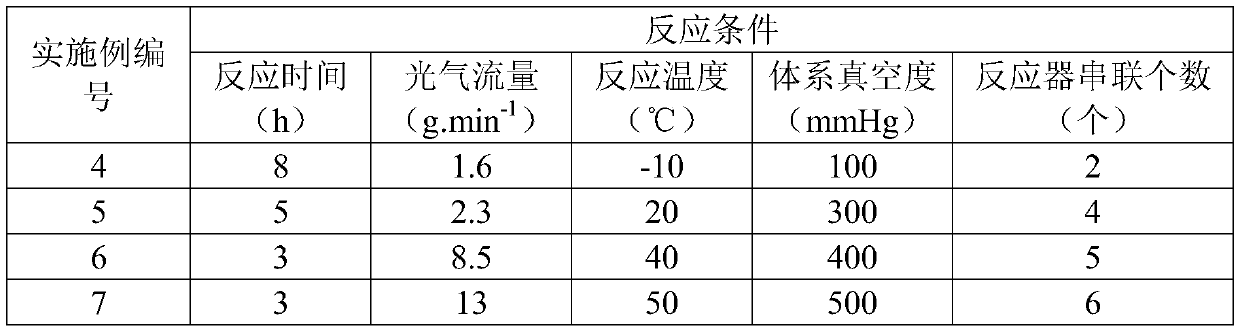
[0052] (1) add isooctyl alcohol 1000g, 800g respectively in first stage, second stage reactor, phosgene passes through the reactor of two stages in series successively, reacts with the isooctyl alcohol in described reactor, makes chloroformic acid -2-Ethylhexyl ester; control the reaction temperature in each stage of reactor to be 10°C, the flow rate of phosgene to be 14g / min, and the degree of vacuum to be 200mmHg;
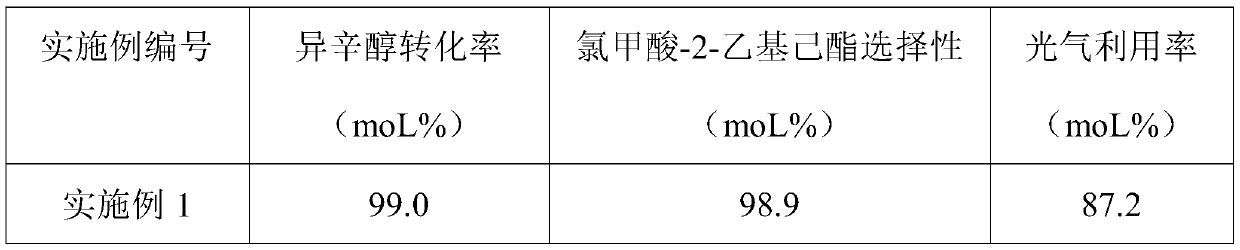
[0053] After the phosgene passes through the second-stage reaction kettle, it is passed into the sodium hydroxide solution for tail gas absorption. When the conversion rate of isooctyl alcohol in the first-stage reaction kettle is >99wt%, stop feeding phosgene, and directly inject the phosgene into the second-stage reaction kettle. Feed phosgene, and so ...
Embodiment 2
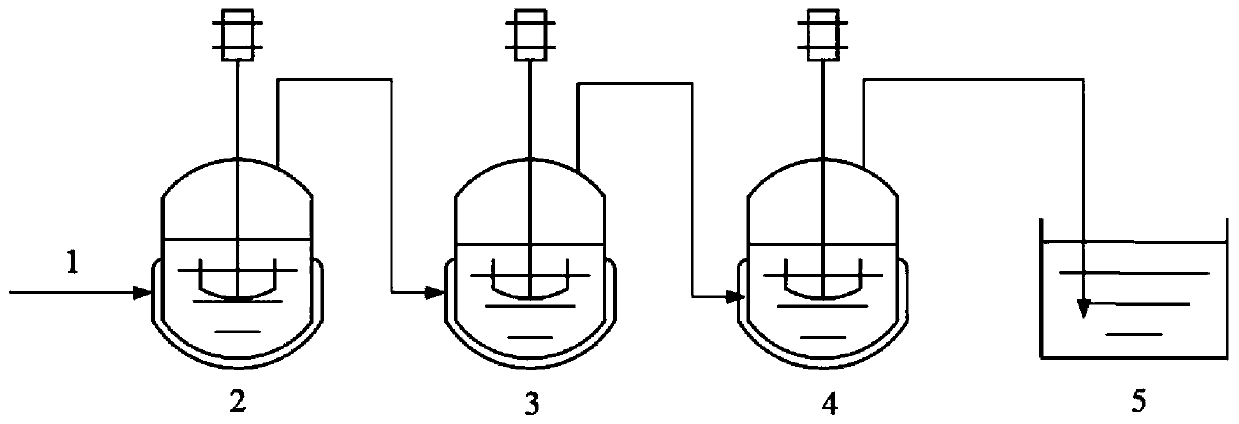
[0056] The present embodiment provides a method for preparing 2-ethylhexyl chloroformate, the method uses a three-stage series reaction kettle, and its schematic flow diagram is as follows figure 1 As shown, it specifically includes the following steps:
[0057] (1) Add isooctyl alcohol 1000g, 800g and 800g to the first-stage reactor 2, the second-stage reactor 3, and the third-stage reactor 4 respectively, and the phosgene passes through the three-stage successively through the phosgene feeding pipeline 1 Reactors connected in series react with the isooctanol in the reactor to produce chloroformic acid-2-ethylhexyl; controlling the reaction temperature in each grade of reactor is 0°C, and the flow rate of phosgene is 3.8g / min, Vacuum degree is 450mmHg;
[0058] Phosgene is passed into the potassium hydroxide solution of tail gas absorption tank 5 after the third stage reaction kettle 3 and carries out tail gas absorption, stops feeding phosgene when the isooctyl alcohol conv...
Embodiment 3
[0061] The present embodiment provides a kind of method for preparing 2-ethylhexyl chloroformate, described method adopts the reactor of four stages in series, specifically comprises the following steps:
[0062] (1) Add isooctyl alcohol 1000g, 800g, 800g, 800g to the first stage, second stage, third stage, and fourth stage reactor respectively, and phosgene passes through the reactor of four stages in series successively, and reacts with the React the isooctyl alcohol in the kettle to prepare 2-ethylhexyl chloroformate; control the reaction temperature in each stage of the reactor to be 30°C, the phosgene flow rate to be 5.9g / min, and the vacuum degree to be 600mmHg;
[0063] After the phosgene is passed through the fourth-stage reactor, it is passed into the sodium carbonate solution for tail gas absorption. When the conversion rate of isooctyl alcohol in the first-stage reactor is >99wt%, stop feeding phosgene, and directly pass it into the second-stage reactor. Enter phosg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com