Synthetic method for capecitabine key intermediate
A capecitabine and synthesis method technology, applied in the field of medicinal chemistry, can solve problems such as increased route cost, and achieve the effects of improved product purity, good quality and high finished product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
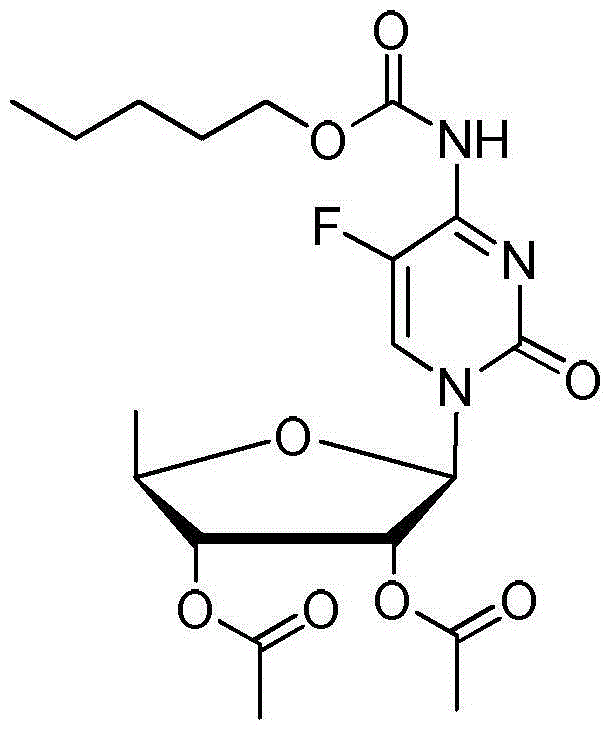
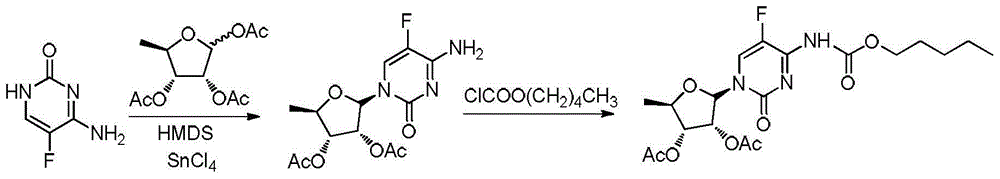
[0034] (1) Mix 100g of 5-fluorocytosine, 600g of chloroform, 123g of sodium carbonate, 200g of water, and 2g of tetrabutylammonium bromide, and add 175g of n-pentyl chloroformate dropwise at 5°C-10°C. After completion, keep warm for the reaction After 1.0 hour, the layers were allowed to stand, and the organic layer was washed with 200 g of water and dried to obtain a chloroform solution ① of pentyl (5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl)carbamate.
[0035](2) Add 201.6g of 1,2,3-tri-O-acetyl-5-deoxy-6-ribofuranose to step ① of step (1), and add 184g of trifluoride dropwise at a temperature of 5°C-10°C After the addition of boroethyl ether, heat up to 35°C-40°C and keep it warm for 3 hours, then drop it back into 413.3g of sodium bicarbonate and 153.3g of water mixture, stir and react for 2h, and filter the organic layer to dry and concentrate it. The concentrated solution was crystallized with 50g methanol and 200g water mixed liquid for 2h, suction filtered and dried to o...
Embodiment 2
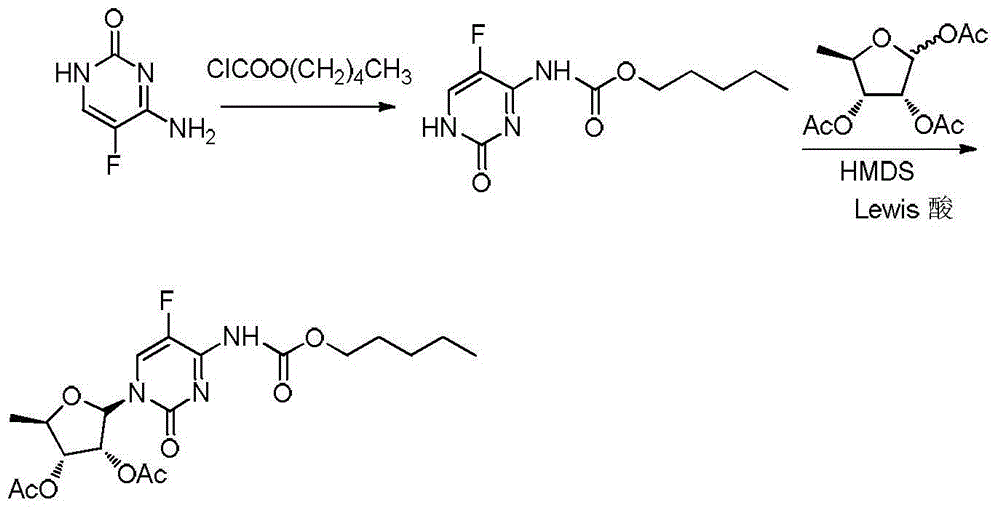
[0037] (1) Mix 100g of 5-fluorocytosine, 800g of chloroform, 117.6g of triethylamine, 2.1g of tetrabutylammonium bisulfate, and 200g of water, add 175g of n-pentyl chloroformate dropwise at 5°C-10°C, add Complete, after 1.0 hours of heat preservation reaction, add dropwise 300g of water to wash, separate layers, and dry to obtain a chloroform solution of (5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl)amyl carbamate ① .
[0038] (2) Add 201.6g of 1,2,3-tri-O-acetyl-5-deoxy-6-ribofuranose to step ① of step (1), and add 184g of trifluoride dropwise at a temperature of 5°C-10°C Boroethyl ether, after completion, heat up to 35°C-40°C and keep it warm for 3 hours, then drop it back into 413.3g of sodium bicarbonate and 153.3g of water mixture, stir and react for 2h, filter the organic layer and dry it, then concentrate it, and then concentrate Mix 50g of methanol and 200g of water for liquid crystallization for 2h, filter with suction and dry to obtain capecitabine intermediate 2',3'-O-...
Embodiment 3
[0040] (1) Mix 100g of 5-fluorocytosine, 600g of chloroform, 123g of sodium carbonate, 200g of water, and 2g of tetrabutylammonium bromide, and add 175g of n-pentyl chloroformate dropwise at 5°C-10°C. After completion, keep warm for the reaction After 1.0 hour, the layers were allowed to stand, and the organic layer was washed with 200 g of water and dried to obtain a chloroform solution ① of pentyl (5-fluoro-2-oxo-1,2-dihydropyrimidin-4-yl)carbamate.
[0041] (2) Add 201.6g of 1,2,3-tri-O-acetyl-5-deoxy-6-ribofuranose to step ① of step (1), and add 185.9g of trichloride at 5°C-10°C Aluminum, complete, heat up to 35°C-40°C and keep it warm for 3 hours, then drop it back into 413.3g of sodium bicarbonate and 153.3g of water mixture, stir for 2 hours, filter the organic layer and dry it, concentrate it, and dilute the concentrated solution Mixed liquid crystallization with 50g methanol and 200g water for 2h, suction filtration and drying to obtain capecitabine intermediate 2',3'...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com