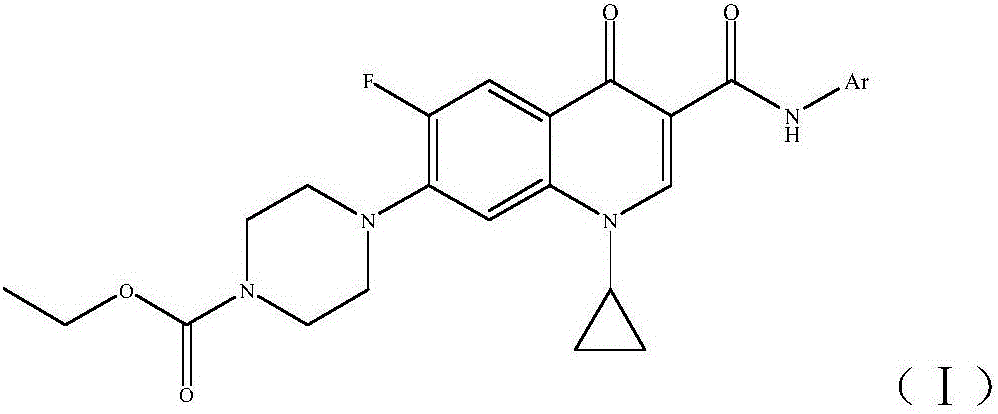
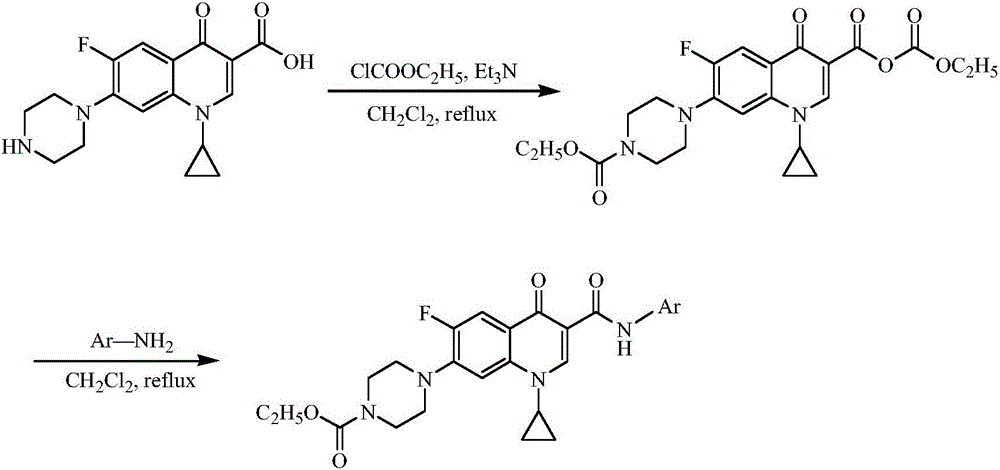
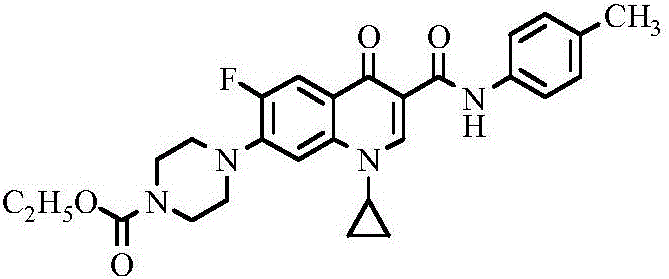
Ciprofloxacin C3 amide derivative, preparation method thereof and application
A technology of amide derivatives and ciprofloxacin, which is applied in the field of medicine, can solve the problems of ciprofloxacin C3 amide derivatives that have not yet had sonodynamic activity, and achieve high antibacterial activity, easy post-processing, and good sonodynamic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] (1) 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-[1-(4-ethoxycarbonyl)piperazinyl]quinoline-3 provided in this example -(N-p-tolyl) formamide, its structural formula is as follows:
[0020]
[0021] (2) Preparation method
[0022] Weigh 3.8582g of ciprofloxacin hydrochloride into a round bottom flask, add 50mL of anhydrous sodium sulfate dried dichloromethane, heat and stir at 38°C, gradually add 20mmol of anhydrous sodium sulfate dried triethylamine dropwise To the reaction solution, adjust the pH of the reaction solution to 7.5-8.5. Then, within half an hour, 15 mmol of ethyl chloroformate was slowly added dropwise to the reaction liquid, and stirred under reflux at 20° C. until the reaction liquid became clear. Add an appropriate amount of triethylamine dropwise to the reaction solution to make the pH 7.5-8.5, then add 10 mmol of p-aminotoluene, and continue to reflux for half an hour. After the reaction solution was cooled, it was distilled under reduced pressur...
Embodiment 2
[0027] (1) 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-[1-(4-ethoxycarbonyl)piperazinyl]quinoline-3 provided in this example -(N-p-chlorophenyl) formamide, its structural formula is:
[0028]
[0029] (2) Preparation method
[0030] Weigh 3.8582g of ciprofloxacin hydrochloride into a round bottom flask, add 50mL of anhydrous sodium sulfate dried dichloromethane, heat and stir at 38°C, gradually add 20mmol of anhydrous sodium sulfate dried triethylamine dropwise To the reaction solution, adjust the pH of the reaction solution to 7.5-8.5. Then, within half an hour, 15 mmol of ethyl chloroformate was slowly added dropwise to the reaction liquid, and stirred under reflux at 20° C. until the reaction liquid became clear. Add an appropriate amount of triethylamine dropwise to the reaction solution to make the pH 7.5-8.5, then add 10 mmol of p-aminochlorobenzene, and continue to reflux for half an hour. After the reaction liquid was cooled, the liquid was distilled under reduced...
Embodiment 3
[0035] (1) 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-[1-(4-ethoxycarbonyl)piperazinyl]quinoline-3 provided in this example -(N-methoxyphenyl) formamide, its structural formula is:
[0036]
[0037] (2) Preparation method
[0038] Weigh 3.8582g of ciprofloxacin hydrochloride into a round bottom flask, add 50mL of anhydrous sodium sulfate dried dichloromethane, heat and stir at 38°C, gradually add 20mmol of anhydrous sodium sulfate dried triethylamine dropwise To the reaction solution, adjust the pH of the reaction solution to 7.5-8.5. Then, within half an hour, 15 mmol of ethyl chloroformate was slowly added dropwise to the reaction liquid, and stirred under reflux at 20° C. until the reaction liquid became clear. Add an appropriate amount of triethylamine dropwise to the reaction solution to make the pH 7.5-8.5, then add 10 mmol of m-methoxyaniline, and continue to reflux for half an hour. After the reaction liquid was cooled, the liquid was distilled under reduced pres...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com