Preparation method of chiral intermediate for synthesizing statins
A technology for the synthesis of chiral intermediates and drugs, applied in organic chemistry and other directions, can solve the problems of decreased purity, low production efficiency, large pollution, etc., to achieve stable reaction process, improve production efficiency, and solve the effect of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] This example is a control group (refer to US20070037979)
[0056] first step:
[0057]
[0058] Put 55.28g (0.2mol) of (3R)-3-[(tert-butyldimethylsilyl)oxy]-glutaric acid monomethyl ester (9) and 100ml tetrahydrofuran into a 500ml three-necked reaction flask, and cool down to -20 Below ℃, 30.36 g (0.3 mol) of triethylamine was added. Continue to cool down to below -50°C, add 32.56g of ethyl chloroformate (0.3mol) dropwise, drop below -30°C, and keep warm for 1.5 to 2 hours.
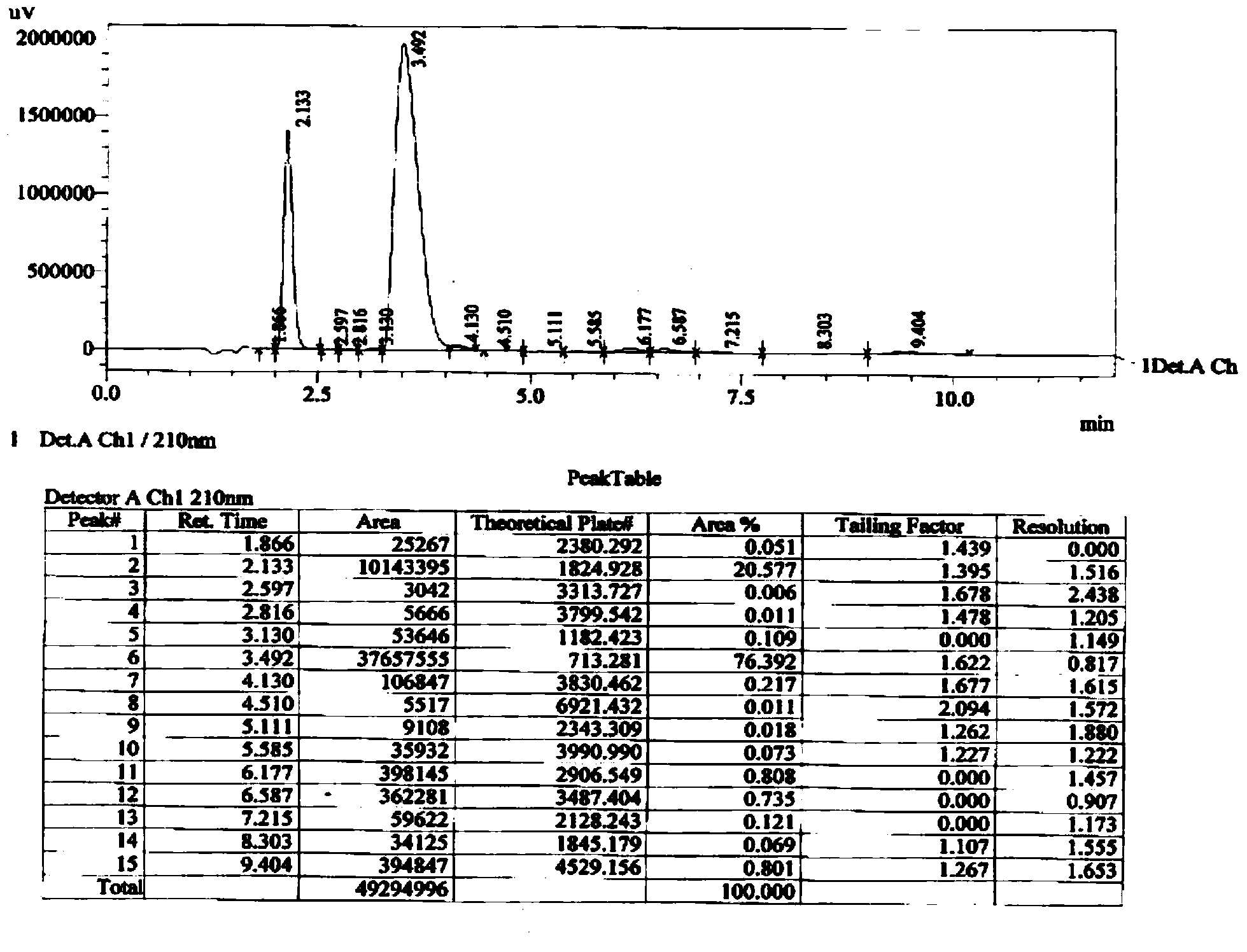
[0059] After the heat preservation is over, add 50ml of water, stir for 5-10 minutes, separate layers, extract the aqueous layer with 50ml of ethyl acetate once, combine the organic phases, wash with 50ml*2 saturated NaHCO3 (aq), and then wash once with 50ml of saturated saline, 10g Dry over anhydrous magnesium sulfate. Filter and evaporate to dryness to obtain 1,5-carboethoxymethyl-(3S)-3-[(tert-butyldimethylsilyl)oxy]-glutaric acid diester 65.6 g. Yield 94.2%.
[0060] Step two:
[0061]...
Embodiment 2
[0073] first step:
[0074]
[0075] Put 55.28g (0.2mol) of (3R)-3-[(tert-butyldimethylsilyl)oxy]-glutaric acid monomethyl ester (9) and 100ml of toluene into a 500ml three-necked reaction flask, and stir at room temperature. 30.36 g (0.3 mol) of triethylamine was added. Cool down to -30~-25°C, add dropwise a solution of 52.40g p-nitrophenyl chloroformate (7) (0.26mol) and 100ml toluene, and keep at -30~-25°C for 1.5~2h after dropping.
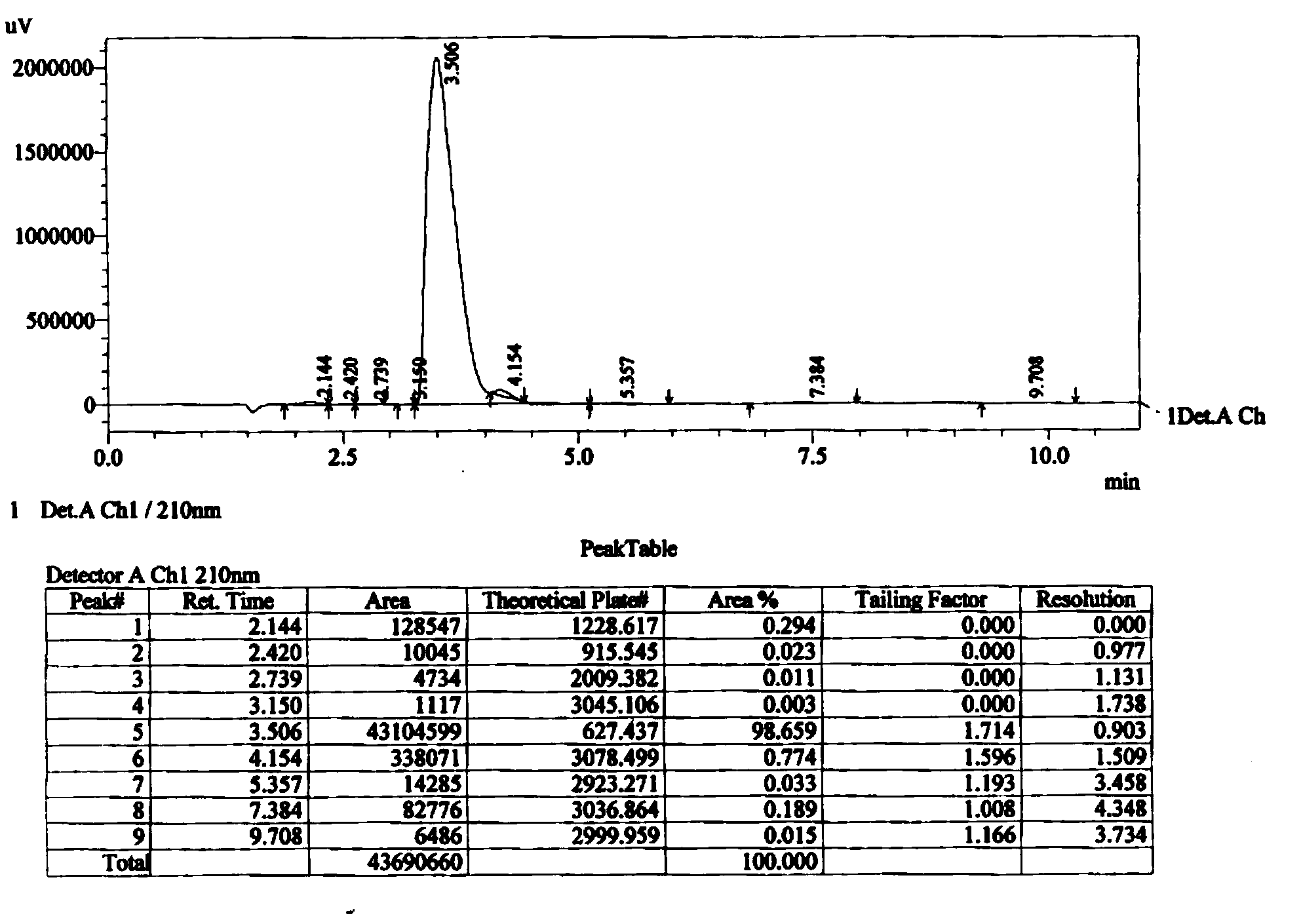
[0076] Add 50ml of water, stir to dissolve the salt produced by the reaction, separate the layers, extract the aqueous layer with 50ml of ethyl acetate once, combine the organic phases, and use 50ml of saturated NaHCO 3 (aq) and 50ml of saturated brine were washed once respectively, and dried with 10g of anhydrous magnesium sulfate. Filtration and evaporation to dryness gave 86.5 g of 1,5-p-nitrophenylcarboxymethyl-(3S)-3-[(tert-butyldimethylsilyl)oxy]-glutaric acid diester (11), The yield is 98.0%.
[0077] Step two:
[0078]
[00...
Embodiment 3
[0084] first step:
[0085] Put 95.54g (0.3mol) of (3R)-3-[(tert-butyldimethylsilyl)oxy]-glutaric acid mono-tert-butyl ester (10) and 150ml of ethyl acetate into a 1000ml liquid-sealed device In a three-necked reaction flask, 82.91 g (0.6 mol) of anhydrous potassium carbonate was added under stirring at room temperature. Control the temperature at 15-20°C, add dropwise a solution of 90.7g p-nitrophenyl chloroformate (7) (0.45mol) and 150ml ethyl acetate, and keep warm at 15-20°C for 1.5-2h after dropping.
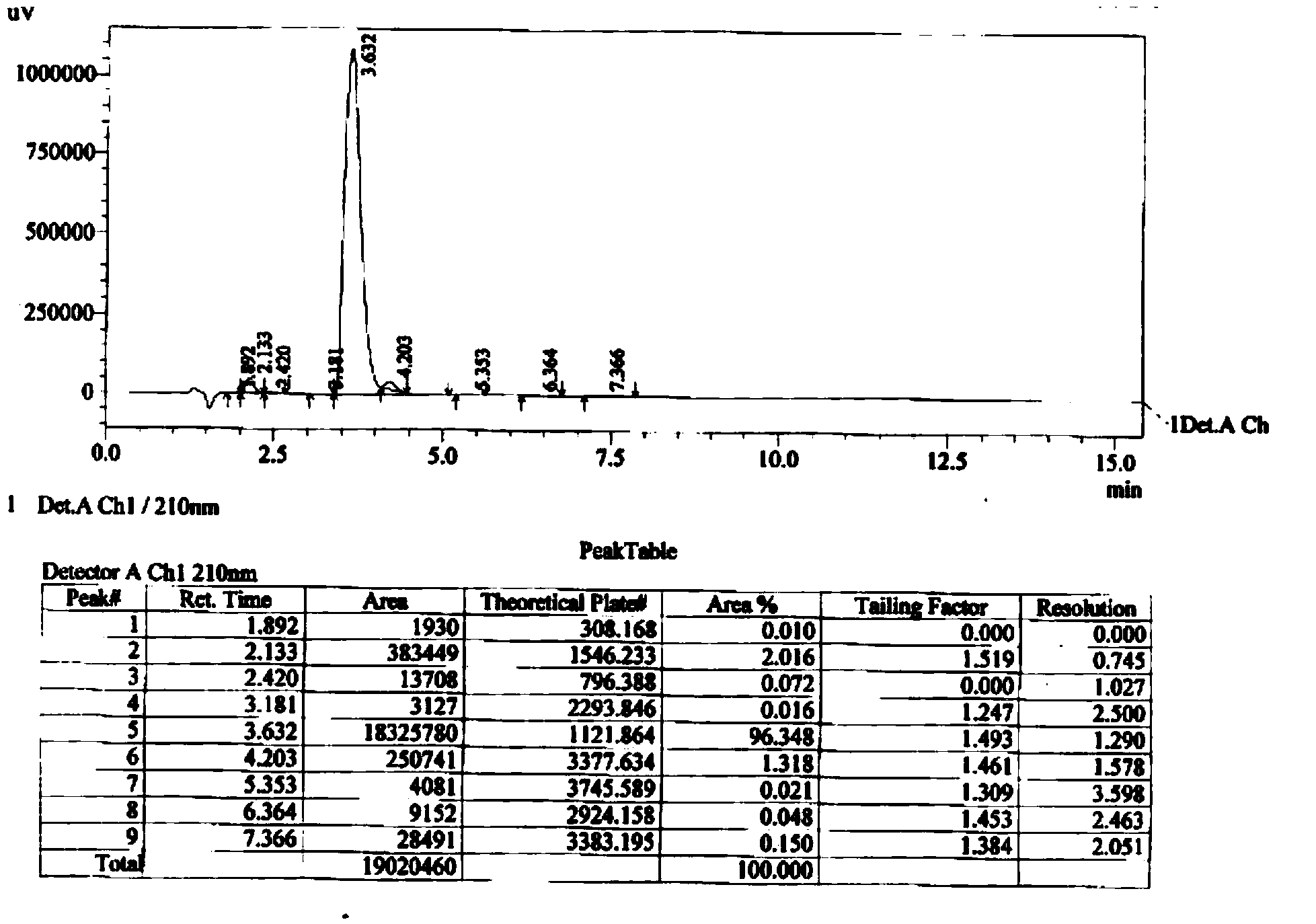
[0086] Add 75ml of water, stir to normal temperature, separate the layers, extract the aqueous layer with 75ml of ethyl acetate once, combine the organic phases, wash with 75ml of saturated NaHCO3 (aq), 75ml of saturated brine, and dry with 15g of anhydrous magnesium sulfate. Filtrate and evaporate to dryness to obtain 143.4 g of 1,5-p-nitrophenylcarboxyt-butyl-(3S)-3-[(tert-butyldimethylsilyl)oxy]-glutaric acid diester (12) , yield 98.8%. Step two:
[0087]
[008...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com