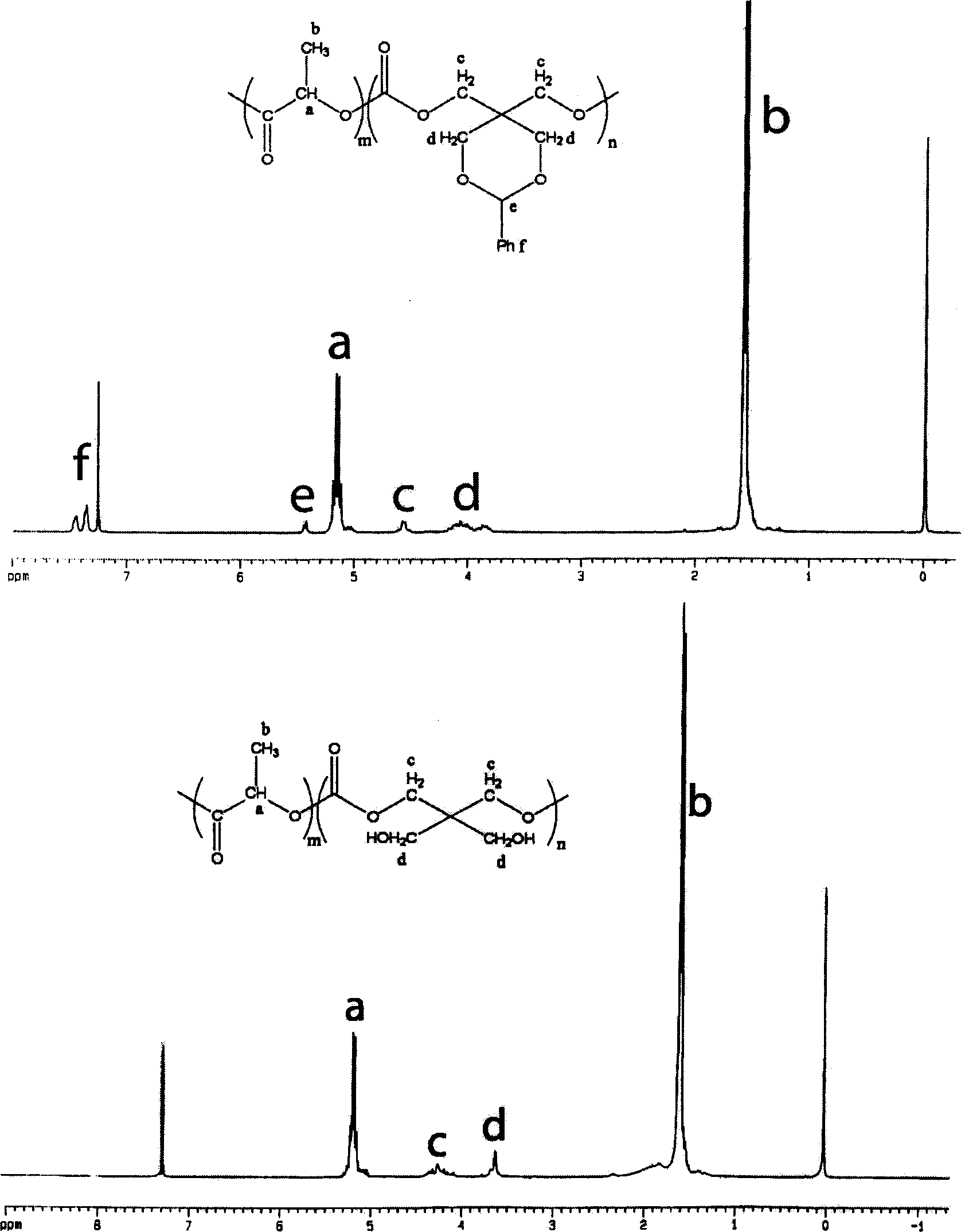
Cyclic aliphatic acid ester carbonate, its polymer, synthesis method and uses thereof
A technology of cyclic aliphatic and aliphatic carbonates, which is applied in the field of biomedical polymer materials, can solve problems such as unsatisfactory biodegradation performance, difficulty in bonding drugs, and limited applications, and achieve low raw material prices and good hydrophilic/hydrophobic properties , high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Synthesis of 2-phenyl-5,5-dimethylol-1,3-dioxane
[0031] Dissolve 68g (0.50mol) of pentaerythritol in 500ml of water, add 53g (0.50mol) of benzaldehyde and 2.5ml of concentrated hydrochloric acid, stir vigorously at room temperature for 5h, let it stand for 24h, wash the crude product with distilled water three times, and then successively wash it with sodium carbonate solution Recrystallized from toluene to obtain 87 g (0.39 mol) of 2-phenyl-5,5-dimethylol-1,3-dioxane as white crystals. Yield 78%.
Embodiment 2
[0032] Example 2: Synthesis of 2,2-dimethyl-5,5-dimethylol-1,3-dioxane
[0033] Dissolve 50g (0.36mol) of pentaerythritol and 0.61g (0.0032mol) of p-toluenesulfonic acid in 500ml of acetone, then add 55.4ml (0.36mol) of 2,2-dimethoxypropane, stir at room temperature for 24h, then add 5ml of ammonia water / ethyl acetate solution (50:50). Concentrate under reduced pressure, add 500ml of dichloromethane, extract with water, collect the organic layer, dry over magnesium sulfate, evaporate the solvent to obtain the product white crystals, namely 2,2-dimethyl-5,5-dimethylol-1 , 3-dioxane. Yield 62%.
Embodiment 3
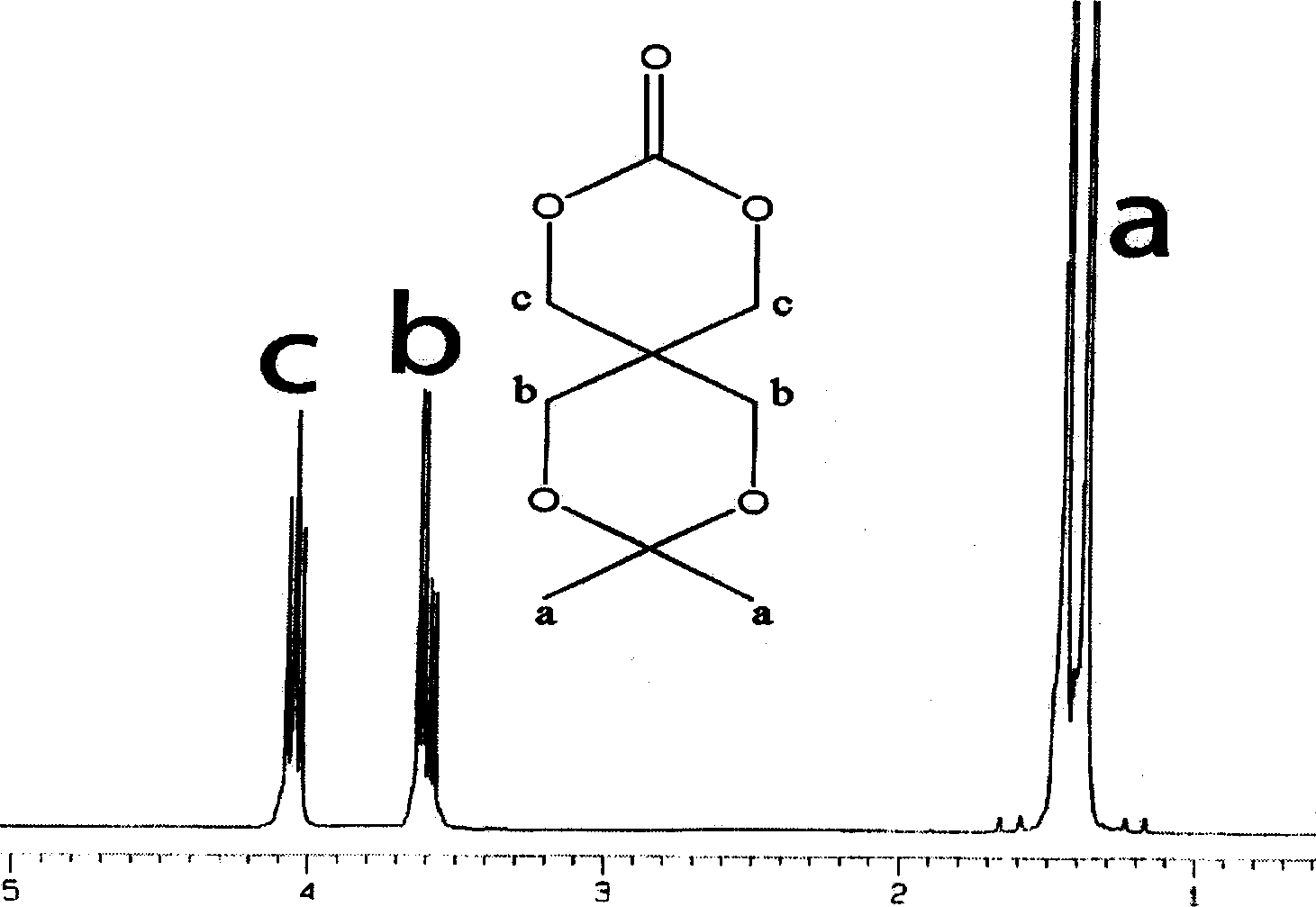
[0034] Example 3: Synthesis of carbonate monomer 9-phenyl-2,4,8,10-tetraoxaspiro[5,5]undecane-3-one
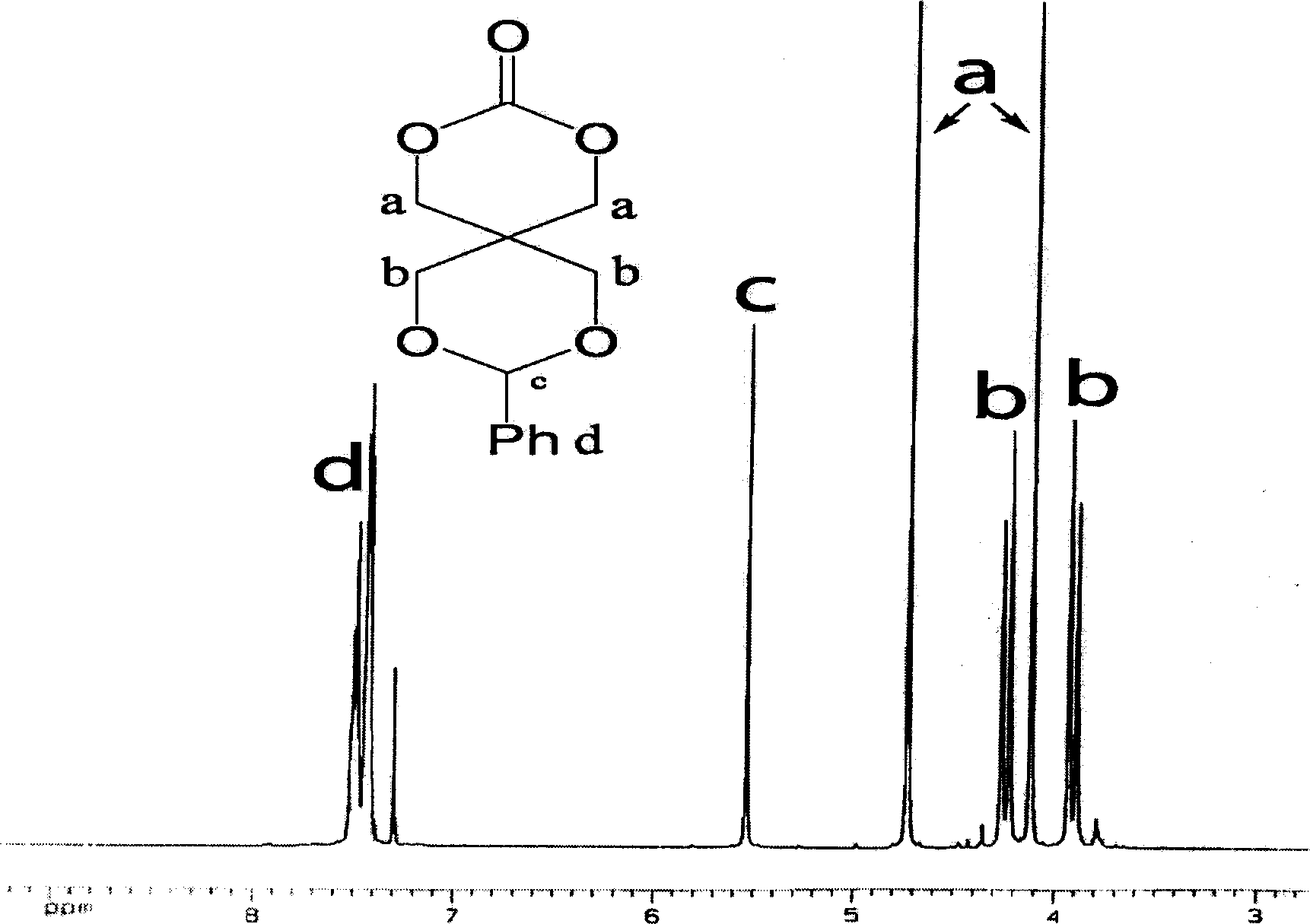
[0035] Dissolve 9.5g (0.042mol) of 2-phenyl-5,5-dimethylol-1,3-dioxane and 8.1ml (0.082mol) of ethyl chloroformate in 400ml of tetrahydrofuran at 0°C Slowly add 12ml (0.086mol) triethylamine, react at room temperature for 2h, filter off the generated triethylamine hydrochloride, concentrate the filtrate under reduced pressure, and recrystallize with tetrahydrofuran / ether to obtain white crystals, namely 9-phenyl- 2,4,8,10-tetraoxaspiro[5,5]undecan-3-one. That 1 See attached for H NMR spectrum figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com