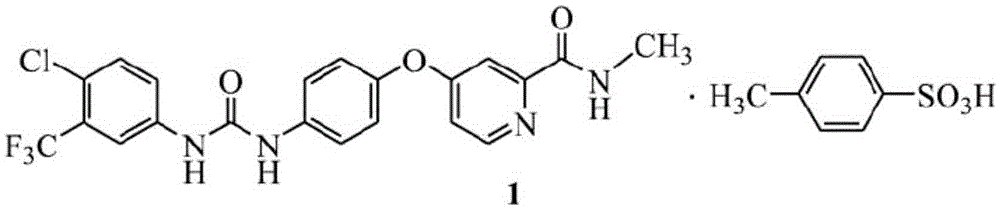
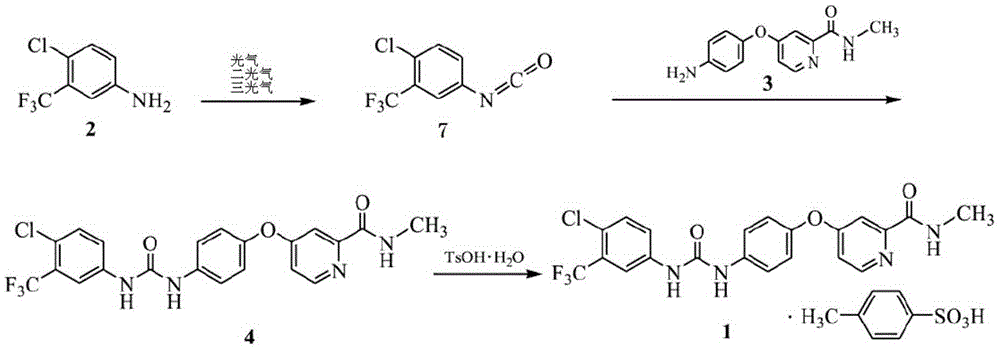
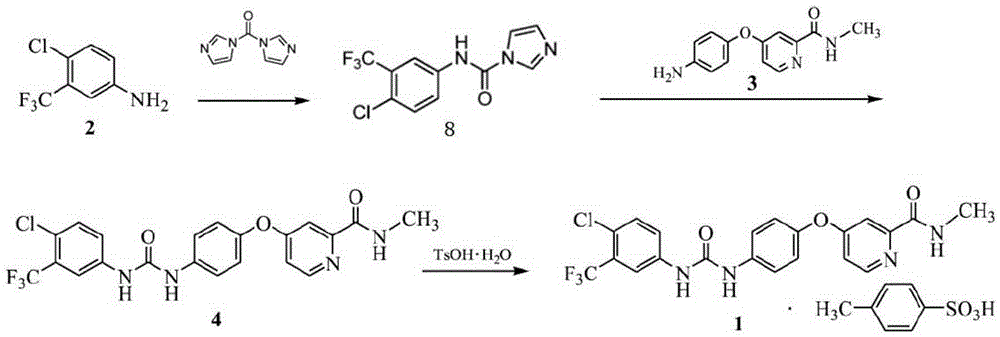
Preparation method of sorafenib tosylate
A technology of sorafenib p-toluenesulfonate and p-toluenesulfonic acid is applied in the field of preparation of sorafenib p-toluenesulfonate, can solve the problems of poor stability, cumbersome reaction operation, long reaction time and the like, and achieves the reaction step Less, safe process, simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Synthesis of (4-chloro-3-trifluoromethylaniline)-propenyl formate (compound 6)
[0033] Triethylamine (42ml, 300mmol) and 4-chloro-3-trifluoromethylaniline (compound 2) (19.6g, 100mmol) were added to 500mL of dichloromethane, and the temperature during the dropping process was controlled below 5°C, and Stir at 0°C~5°C until clear; add propylene chloroformate (compound 5) (11.7ml, 110mmol) dropwise, control the temperature of the dropping process below 5°C; stir the reaction mixture at room temperature for 2 hours, and use brine for the reaction solution Washing (4×300mL), anhydrous Na 2 SO 4 Dry, filter, concentrate the filtrate to a crude product, crystallize with ethyl acetate: n-heptane (1:2) solution, filter with suction, and dry to obtain (4-chloro-3-trifluoromethylaniline)-propenyl formate (Compound 6 ) 25.6g, yield 91.6%, purity 99.3% (HPLC method).
Embodiment 2
[0034] Example 2: Synthesis of (4-chloro-3-trifluoromethylaniline)-propenyl formate (compound 6)
[0035] Add sodium hydroxide aqueous solution (100mL, 2M) and 4-chloro-3-trifluoromethylaniline (compound 2) (19.6g, 100mmol) to 500mL ethyl acetate, and control the temperature of the dropping process below 5°C, And stir at 0℃~5℃ for 30min; add propylene chloroformate (compound 5) (15ml, 140mmol) dropwise, control the temperature of the dropping process below 5℃; stir the reaction mixture at room temperature for 3 hours, then separate the mixture, The aqueous phase was extracted with ethyl acetate (4×300 mL), the organic phases were combined, washed with brine (3×400 mL), anhydrous Na 2 SO 4 Dry, filter, concentrate the filtrate to a crude product, crystallize with ethyl acetate: n-heptane (1:2) solution, filter with suction, and dry to obtain (4-chloro-3-trifluoromethylaniline)-propenyl formate (Compound 6 ) 25.2g, yield 90.3%, purity 99.2% (HPLC method).
Embodiment 3
[0036] Example 3: Synthesis of (4-chloro-3-trifluoromethylaniline)-propenyl formate (compound 6)
[0037] Add N,N-diisopropylethylamine (33ml, 200mmol) and 4-chloro-3-trifluoromethylaniline (compound 2) (19.6g, 100mmol) to 500mL ethyl acetate to control the dropping process The temperature is below 5℃, and stir at 0℃~5℃ until it is clear; propylene chloroformate (compound 5) (16ml, 150mmol) is added dropwise, and the temperature of the dripping process is controlled below 5℃; the reaction mixture is stirred at room temperature 2 Hours, the reaction solution was washed with brine (4×300mL), anhydrous Na 2 SO 4 Dry, filter, concentrate the filtrate to a crude product, crystallize with ethyl acetate: n-heptane (1:2) solution, filter with suction, and dry to obtain (4-chloro-3-trifluoromethylaniline)-propenyl formate (Compound 6 ) 25.5g, yield 91.3%, purity 99.2% (HPLC method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com