Preparation method of tulathromycin
A technology of telamycin and methyl azithromycin, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of epoxidation with many impurities, low yield of double-protected intermediates, and difficulty in purification, etc. problem, to achieve high yield, easy to protect, and beneficial to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
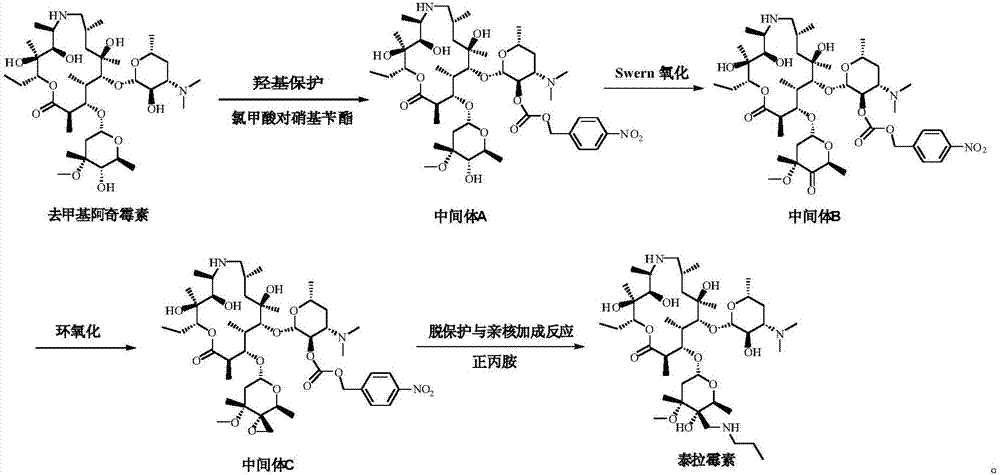
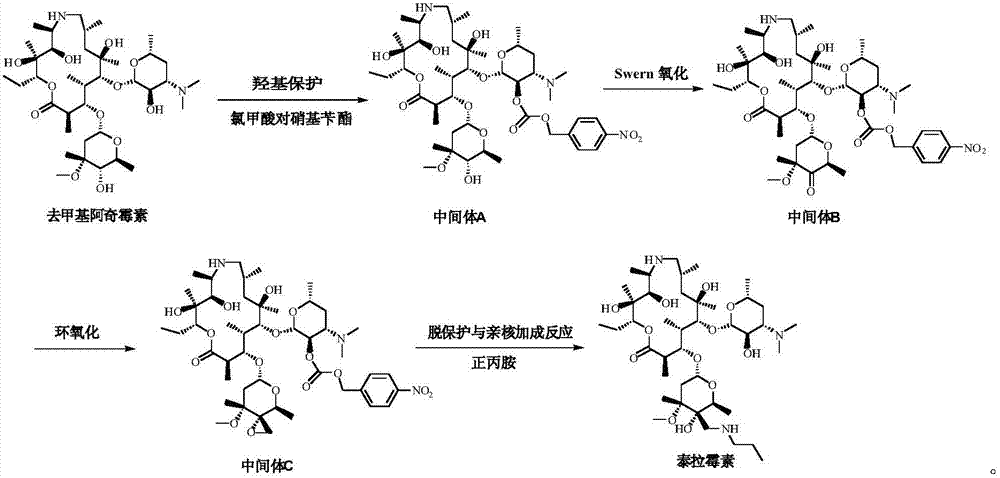
[0057] (1) Preparation of Intermediate A
[0058] Add 140mL dichloromethane and 17g (23.1mmol) demethylazithromycin to the reaction flask, control the temperature at -5~0°C, add 6.0g (27.6mmol) p-nitrobenzyl chloroformate, add dropwise 3.0g (23.1mmol) ) diisopropylethylamine, reacted for 3h to obtain a dichloromethane solution of intermediate A, and directly carried out the next step reaction.
[0059] (2) Preparation of Intermediate B
[0060] Add 32 g (409.5 mol) of dimethyl sulfoxide to the obtained solution of intermediate A, lower the temperature to -80~-70° C., add 16.2 g (77.0 mmol) of trifluoroacetic anhydride dropwise, and react for 1 hour after dropping. Add 17.2 g (170.0 mmol) of triethylamine and react for 1 hour. After the reaction is complete, add 100 mL of water to the system for extraction three times, distill the organic phase under reduced pressure until the solvent is viscous, add 200 mL of isopropanol to dissolve, and adjust Crystallization temperature 15...
Embodiment 2
[0067] (1) Preparation of Intermediate A
[0068] Add 170mL dichloromethane and 17g (23.1mmol) demethylazithromycin to the reaction flask, control the temperature at -5~0°C, add 6.0g (27.6mmol) p-nitrobenzyl chloroformate, add dropwise 6.5g (34.7mmol) ) tributylamine, reacted for 3h, prepared the dichloromethane solution of intermediate A, and directly carried out the next step reaction.
[0069] (2) Preparation of Intermediate B
[0070] Add 32 g (409.5 mol) of dimethyl sulfoxide to the obtained solution of intermediate A, lower the temperature to -80~-70° C., add 16.2 g (77.0 mmol) of trifluoroacetic anhydride dropwise, and react for 1 hour after dropping. Add 31.4g (170.0mmol) of tributylamine, and react for 1 hour. After the reaction is completed, add 100mL water to the system for extraction three times each time. The organic phase is distilled under reduced pressure until the solvent is viscous. After adding 200mL isopropanol to dissolve, adjust The crystallization temp...
Embodiment 3
[0077] (1) Preparation of Intermediate A
[0078] Add 200mL of dichloromethane and 17g (23.1mmol) of demethylazithromycin to the reaction flask, control the temperature at -5~0°C, add 6.0g (27.6mmol) of p-nitrobenzyl chloroformate, add 3g (34.7mmol) dropwise Triethylamine was reacted for 3 hours to obtain a dichloromethane solution of Intermediate A, which was directly carried out to the next step.
[0079] (2) Preparation of Intermediate B
[0080] Add 32 g (409.5 mol) of dimethyl sulfoxide to the obtained solution of intermediate A, lower the temperature to -80~-70° C., add 16.2 g (77.0 mmol) of trifluoroacetic anhydride dropwise, and react for 1 hour after dropping. Add 17.2 g (170.0 mmol) of triethylamine and react for 1 hour. After the reaction is complete, add 100 mL of water to the system for extraction three times. The organic phase is distilled under reduced pressure until it becomes viscous. After adding 200 mL of ethanol to dissolve, adjust the crystallization temp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com